Precision Antiplatelet Therapy after Percutaneous Coronary Intervention (Precision PCI) Registry - Informing optimal antiplatelet strategies
- PMID: 39150361
- PMCID: PMC11328342
- DOI: 10.1111/cts.70004
Precision Antiplatelet Therapy after Percutaneous Coronary Intervention (Precision PCI) Registry - Informing optimal antiplatelet strategies
Abstract
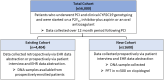
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor (clopidogrel, prasugrel, or ticagrelor) is indicated after percutaneous coronary intervention (PCI) to reduce the risk of atherothrombotic events. Approximately 30% of the US population has a CYP2C19 no-function allele that reduces the effectiveness of clopidogrel, but not prasugrel or ticagrelor, after PCI. We have shown improved outcomes with the integration of CYP2C19 genotyping into clinical care to guide the selection of prasugrel or ticagrelor in CYP2C19 no-function allele carriers. However, the influence of patient-specific demographic, clinical, and other genetic factors on outcomes with genotype-guided DAPT has not been defined. In addition, the impact of genotype-guided de-escalation from prasugrel or ticagrelor to clopidogrel in patients without a CYP2C19 no-function allele has not been investigated in a diverse, real-world clinical setting. The Precision Antiplatelet Therapy after Percutaneous Coronary Intervention (Precision PCI) Registry is a multicenter US registry of patients who underwent PCI and clinical CYP2C19 testing. The registry is enrolling a diverse population, assessing atherothrombotic and bleeding events over 12 months, collecting DNA samples, and conducting platelet function testing in a subset of patients. The registry aims to define the influence of African ancestry and other patient-specific factors on clinical outcomes with CYP2C19-guided DAPT, evaluate the safety and effectiveness of CYP2C19-guided DAPT de-escalation following PCI in a real-world setting, and identify additional genetic influences of clopidogrel response after PCI, with the ultimate goal of establishing optimal strategies for individualized antiplatelet therapy that improves outcomes in a diverse, real-world population.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
F.F. has received consulting fees or honoraria from AstraZeneca; his institution has received research grants from PLx Pharma and the Scott R. MacKenzie Foundation. P.S. has received consulting fees from Medosome Biotech; A.G.W has received consulting fees from Arbor Pharmaceuticals, Bayer KG, Ipsen Pharmaceuticals and Genentech. Her institution has received funding for research for which she served as principal investigator from Merck, Sharpe and Dohme. D.J.A. has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Faraday, Haemonetics, Janssen, Merck, Novartis, NovoNordisk, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Faraday, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co, Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. All other authors declared no competing interests for this work.
Figures

References
-
- Martin SS, Aday AW, Almarzooq ZI, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149:e347‐e913. - PubMed
-
- Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non‐responders compared with clopidogrel in aspirin‐treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166‐1173. - PubMed
-
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577‐2585. - PubMed
-
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001‐2015. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

