Luteinizing Hormone Receptor Mutation (LHRN316S) Causes Abnormal Follicular Development Revealed by Follicle Single-Cell Analysis and CRISPR/Cas9
- PMID: 39150470
- PMCID: PMC11512921
- DOI: 10.1007/s12539-024-00646-7
Luteinizing Hormone Receptor Mutation (LHRN316S) Causes Abnormal Follicular Development Revealed by Follicle Single-Cell Analysis and CRISPR/Cas9
Abstract
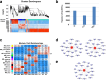
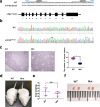
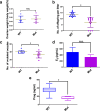
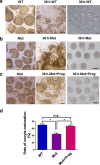
Abnormal interaction between granulosa cells and oocytes causes disordered development of ovarian follicles. However, the interactions between oocytes and cumulus granulosa cells (CGs), oocytes and mural granulosa cells (MGs), and CGs and MGs remain to be fully explored. Using single-cell RNA-sequencing (scRNA-seq), we determined the transcriptional profiles of oocytes, CGs and MGs in antral follicles. Analysis of scRNA-seq data revealed that CGs may regulate follicular development through the BMP15-KITL-KIT-PI3K-ARF6 pathway with elevated expression of luteinizing hormone receptor (LHR). Because internalization of the LHR is regulated by Arf6, we constructed LHRN316S mice by CRISPR/Cas9 to further explore mechanisms of follicular development and novel treatment strategies for female infertility. Ovaries of LHRN316S mice exhibited reduced numbers of corpora lutea and ovulation. The LHRN316S mice had a reduced rate of oocyte maturation in vitro and decreased serum progesterone levels. Mating LHRN316S female mice with ICR wild type male mice revealed that the infertility rate of LHRN316S mice was 21.4% (3/14). Litter sizes from LHRN316S mice were smaller than those from control wild type female mice. The oocytes from LHRN316S mice had an increased rate of maturation in vitro after progesterone administration in vitro. Furthermore, progesterone treated LHRN316S mice produced offspring numbers per litter equivalent to WT mice. These findings provide key insights into cellular interactions in ovarian follicles and provide important clues for infertility treatment.
Keywords: Follicle; Granulosa cells; LHRN316S; Oocytes; Progesterone; Single cell RNA-seq.
© 2024. The Author(s).
Conflict of interest statement
There is no disclosure of potential conflicts of interest.
Figures


Similar articles
-
Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells.Biol Reprod. 1997 Apr;56(4):976-84. doi: 10.1095/biolreprod56.4.976. Biol Reprod. 1997. PMID: 9096881
-
Localization of luteinizing hormone receptor protein in the human ovary.Mol Hum Reprod. 2014 Sep;20(9):844-9. doi: 10.1093/molehr/gau041. Epub 2014 May 29. Mol Hum Reprod. 2014. PMID: 24874553
-
Immunohistochemical Localization of Luteinizing Hormone Receptor in the Cyclic Gilt Ovary.Anat Histol Embryol. 2017 Feb;46(1):94-100. doi: 10.1111/ahe.12255. Epub 2016 Sep 9. Anat Histol Embryol. 2017. PMID: 27611647
-
Intrafollicular content of luteinizing hormone receptor, alpha-inhibin, and aromatase in relation to follicular growth, estrous cycle stage, and oocyte competence for in vitro maturation in the mare.Biol Reprod. 1999 May;60(5):1120-7. doi: 10.1095/biolreprod60.5.1120. Biol Reprod. 1999. PMID: 10208973
-
Ovarian follicular and luteal physiology.Int Rev Physiol. 1980;22:117-201. Int Rev Physiol. 1980. PMID: 6248477 Review.
References
-
- Brunet S, Maro B (2007) Germinal vesicle position and meiotic maturation in mouse oocyte. Reproduction 133:1069–1072. 10.1530/REP-07-0036 - PubMed
-
- Gittens JE, Barr KJ, Vanderhyden BC et al (2005) Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J Cell Sci 118:113–122. 10.1242/jcs.01587 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

