Remote Neuroinflammation in Newly Diagnosed Glioblastoma Correlates with Unfavorable Clinical Outcome
- PMID: 39150564
- PMCID: PMC11474166
- DOI: 10.1158/1078-0432.CCR-24-1563
Remote Neuroinflammation in Newly Diagnosed Glioblastoma Correlates with Unfavorable Clinical Outcome
Abstract
Purpose: Current therapy strategies still provide only limited success in the treatment of glioblastoma, the most frequent primary brain tumor in adults. In addition to the characterization of the tumor microenvironment, global changes in the brain of patients with glioblastoma have been described. However, the impact and molecular signature of neuroinflammation distant of the primary tumor site have not yet been thoroughly elucidated.
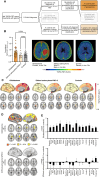
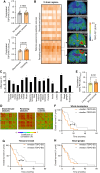
Experimental design: We performed translocator protein (TSPO)-PET in patients with newly diagnosed glioblastoma (n = 41), astrocytoma WHO grade 2 (n = 7), and healthy controls (n = 20) and compared TSPO-PET signals of the non-lesion (i.e., contralateral) hemisphere. Back-translation into syngeneic SB28 glioblastoma mice was used to characterize Pet alterations on a cellular level. Ultimately, multiplex gene expression analyses served to profile immune cells in remote brain.
Results: Our study revealed elevated TSPO-PET signals in contralateral hemispheres of patients with newly diagnosed glioblastoma compared to healthy controls. Contralateral TSPO was associated with persisting epileptic seizures and shorter overall survival independent of the tumor phenotype. Back-translation into syngeneic glioblastoma mice pinpointed myeloid cells as the predominant source of contralateral TSPO-PET signal increases and identified a complex immune signature characterized by myeloid cell activation and immunosuppression in distant brain regions.
Conclusions: Neuroinflammation within the contralateral hemisphere can be detected with TSPO-PET imaging and associates with poor outcome in patients with newly diagnosed glioblastoma. The molecular signature of remote neuroinflammation promotes the evaluation of immunomodulatory strategies in patients with detrimental whole brain inflammation as reflected by high TSPO expression.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
V. Zenatti reports grants from Alzheimer’s Association Grant through the AD Strategic Fund (ADSF-21-831226-C) during the conduct of the study. A. Holzgreve reports personal fees from ABX advanced biochemical compounds outside the submitted work. M. Groß reports grants from Ludwig-Maximilian-University during the conduct of the study. J.C. Tonn reports grants from DFG during the conduct of the study as well as grants from Novocure, non-financial support from Munich Surgical Imaging, and personal fees from Servier and Novartis outside the submitted work. N.L. Albert reports grants from German Research Council during the conduct of the study as well as grants and personal fees from Telix Pharmaceuticals; personal fees from Novartis, Advanced Accelerator Applications, OncLive, and Servier; and grants from Thomas Kirch Foundation and Novocure outside the submitted work. M. Brendel reports grants from German Research Foundation during the conduct of the study as well as personal fees from Roche, GE Healthcare, and MIAC; grants, personal fees, and non-financial support from Life Molecular Imaging; and grants from MJFF outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. . Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci 2021;24:595–610. - PubMed

