Obicetrapib-the Rebirth of CETP Inhibitors?
- PMID: 39150671
- PMCID: PMC11393031
- DOI: 10.1007/s11883-024-01231-5
Obicetrapib-the Rebirth of CETP Inhibitors?
Abstract
Purpose of review: To provide perspective on the current development status, and potential future role, of obicetrapib, a third-generation cholesterylester transfer protein (CETP) inhibitor. Obicetrapib has received recent attention following positive Phase II clinical trial data and initiation of Phase III trials for the treatment of dyslipidemia and atherosclerotic cardiovascular disease (ASCVD).
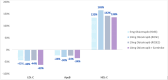
Recent findings: The ROSE and ROSE2 trials are Phase II studies that examined the lipid lowering effects of obicetrapib in patients on pre-existing high-intensity statin therapy. Obicetrapib significantly reduced key dyslipidemia biomarkers including low density lipoprotein cholesterol (LDL-C), Apolipoprotein B (Apo B), and non-high-density lipoprotein cholesterol (non-HDL-C) while increasing high-density lipoprotein cholesterol (HDL-C). Four phase III clinical trials, including a cardiovascular outcomes trial, are ongoing. Preliminary data for obicetrapib shows favorable effects on dyslipidemia, which could theoretically lead to a decrease in ASCVD clinical events. Short-term safety data in preliminary studies shows no significant safety signals.
Keywords: CETP; Cholesterylester transfer protein inhibitor; Lipid lowering; Low-density lipoprotein; Obicetrapib.
© 2024. The Author(s).
Conflict of interest statement
Chang – No disclosures.
Laffin – Dr. Laffin is a consultant and/or served on steering committees for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, Idorsia, New Amsterdam, and Crispr Therapeutics; has received research funding from AstraZeneca; and has ownership interest in LucidAct Health and Gordy Health.
Sarraju – Dr. Sarraju is the principal investigator of the TANDEM trial of obicetrapib. He receives no personal renumeration for this role as it paid to the C5 Research, the academic research organization of the Cleveland Clinic’s Heart, Vascular, and Thoracic Institute.
Nissen – Dr. Nissen is the study chair for the TANDEM trial and a member of the Executive Committee supervising the PREVAIL Trial.
Figures
References
-
- Mortality in the United States: past, present, and future. In: Budget model. University of Pennsylvania. 2016. https://www.budgetmodel.wharton.upenn.edu/issues/2016/1/25/mortality-in-.... Accessed 4 July 2024.
-
- World Health Organization. In: Leading causes of death globally. 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 4 July 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials