Universal cold RNA phase transitions
- PMID: 39150781
- PMCID: PMC11348302
- DOI: 10.1073/pnas.2408313121
Universal cold RNA phase transitions
Abstract
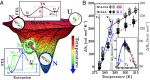
RNA's diversity of structures and functions impacts all life forms since primordia. We use calorimetric force spectroscopy to investigate RNA folding landscapes in previously unexplored low-temperature conditions. We find that Watson-Crick RNA hairpins, the most basic secondary structure elements, undergo a glass-like transition below [Formula: see text]C where the heat capacity abruptly changes and the RNA folds into a diversity of misfolded structures. We hypothesize that an altered RNA biochemistry, determined by sequence-independent ribose-water interactions, outweighs sequence-dependent base pairing. The ubiquitous ribose-water interactions lead to universal RNA phase transitions below TG, such as maximum stability at [Formula: see text]C where water density is maximum, and cold denaturation at [Formula: see text]C. RNA cold biochemistry may have a profound impact on RNA function and evolution.
Keywords: RNA in the cold; RNA phase transitions; cold RNA misfolding; single-RNA force spectroscopy.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

