Vascular Endothelial Growth Factor-B Blockade with CSL346 in Diabetic Kidney Disease: A Phase 2A Randomized Controlled Trial
- PMID: 39150859
- PMCID: PMC11543004
- DOI: 10.1681/ASN.0000000000000438
Vascular Endothelial Growth Factor-B Blockade with CSL346 in Diabetic Kidney Disease: A Phase 2A Randomized Controlled Trial
Abstract
Key Points:
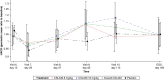
The vascular endothelial growth factor B inhibitor CSL346 (8 or 16 mg/kg q4w) did not reduce urinary albumin-creatinine ratio at week 16 versus placebo in patients with type 2 diabetes mellitus and diabetic kidney disease.
CSL346 was generally well tolerated at both doses; however, CSL346 (16 mg/kg) significantly increased diastolic BP versus placebo.
Background: Increased vascular endothelial growth factor B (VEGF-B) expression in patients with diabetic kidney disease (DKD) is associated with increased lipid deposition in glomerular podocytes. Reducing VEGF-B activity in animal models of DKD using an anti–VEGF-B antibody improved histological evidence of glomerular injury and reduced albuminuria, effects attributed to prevention of ectopic lipid deposition in the kidney. CSL346 is a novel humanized monoclonal antibody that binds VEGF-B with high affinity. Targeting VEGF-B in patients with type 2 diabetes mellitus may improve DKD progression markers.
Methods:
An international, randomized, double-blind, placebo-controlled, phase 2a study (
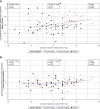
Results: In total, 114 participants were randomized. CSL346 did not significantly reduce UACR compared with placebo at week 16 (combined CSL346 group difference from placebo [95% confidence interval], 4.0% [−14.7 to 26.8]). Furthermore, no effect was seen in participant subgroups (degree of kidney impairment or sodium-glucose cotransporter 2 inhibitor use) or on urinary biomarkers reflecting proximal tubular injury. CSL346 was generally well tolerated; however, diastolic BP was significantly higher with CSL346 16 mg/kg versus placebo from week 2 onward, with differences ranging from +3.8 to +5.3 mm Hg (P = 0.002 at week 16).
Conclusions: CSL346 did not reduce UACR compared with placebo at 16 weeks in participants with type 2 diabetes mellitus and DKD and was associated with an increase in diastolic BP.
Clinical Trial registry name and registration number::
VEGF-B Blockade with the Monoclonal Antibody CSL346 in Subjects with DKD,
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures



References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

