Sarmentosin alleviates doxorubicin-induced cardiotoxicity and ferroptosis via the p62-Keap1-Nrf2 pathway
- PMID: 39150892
- PMCID: PMC11332294
- DOI: 10.1080/13510002.2024.2392329
Sarmentosin alleviates doxorubicin-induced cardiotoxicity and ferroptosis via the p62-Keap1-Nrf2 pathway
Abstract
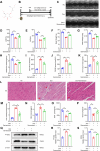
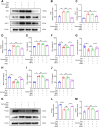
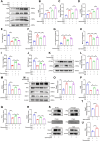
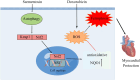
Doxorubicin (Dox) is extensively used as an antitumor agent, but its severe cardiotoxicity significantly limits its clinical use. Current treatments for Dox-induced cardiotoxicity are inadequate, necessitating alternative solutions. This study evaluated the effects of sarmentosin, a compound from Sedum sarmentosum, on Dox-induced cardiotoxicity and dysfunction. Sarmentosin was administered as a pretreatment to both mice and H9c2 cells before Dox exposure. Subsequently, markers of Dox-induced cardiotoxicity and ferroptosis in serum and cell supernatants were measured. Western blot analysis was utilized to detect levels of ferroptosis, oxidative stress, and autophagy proteins. Additionally, echocardiography, hematoxylin-eosin staining, ROS detection, and immunofluorescence techniques were employed to support our findings. Results demonstrated that sarmentosin significantly inhibited iron accumulation, lipid peroxidation, and oxidative stress, thereby reducing Dox-induced ferroptosis and cardiotoxicity in C57BL/6 mice and H9c2 cells. The mechanism involved the activation of autophagy and the Nrf2 signaling pathway. These findings suggest that sarmentosin may prevent Dox-induced cardiotoxicity by mitigating ferroptosis. The study underscores the potential of compounds like sarmentosin in treating Dox-induced cardiotoxicity.
Keywords: Doxorubicin; Sarmentosin; autophagy; cardiotoxicity; ferroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
