A thalamic nucleus reuniens-lateral septum-lateral hypothalamus circuit for comorbid anxiety-like behaviors in chronic itch
- PMID: 39150998
- PMCID: PMC11328909
- DOI: 10.1126/sciadv.adn6272
A thalamic nucleus reuniens-lateral septum-lateral hypothalamus circuit for comorbid anxiety-like behaviors in chronic itch
Abstract
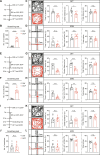
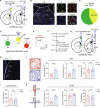
Chronic itch often clinically coexists with anxiety symptoms, creating a vicious cycle of itch-anxiety comorbidities that are difficult to treat. However, the neuronal circuit mechanisms underlying the comorbidity of anxiety in chronic itch remain elusive. Here, we report anxiety-like behaviors in mouse models of chronic itch and identify γ-aminobutyric acid-releasing (GABAergic) neurons in the lateral septum (LS) as the key player in chronic itch-induced anxiety. In addition, chronic itch is accompanied with enhanced activity and synaptic plasticity of excitatory projections from the thalamic nucleus reuniens (Re) onto LS GABAergic neurons. Selective chemogenetic inhibition of the Re → LS circuit notably alleviated chronic itch-induced anxiety, with no impact on anxiety induced by restraint stress. Last, GABAergic neurons in lateral hypothalamus (LH) receive monosynaptic inhibition from LS GABAergic neurons to mediate chronic itch-induced anxiety. These findings underscore the potential significance of the Re → LS → LH pathway in regulating anxiety-like comorbid symptoms associated with chronic itch.
Figures






References
-
- Jafferany M., Davari M. E., Itch and psyche: Psychiatric aspects of pruritus. Int. J. Dermatol. 58, 3–23 (2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

