Magnetically attracting hydrogel reshapes iron metabolism for tissue repair
- PMID: 39151007
- PMCID: PMC11328908
- DOI: 10.1126/sciadv.ado7249
Magnetically attracting hydrogel reshapes iron metabolism for tissue repair
Abstract
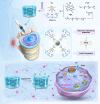
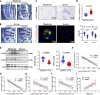
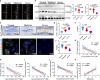
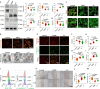
Ferroptosis, caused by disorders of iron metabolism, plays a critical role in various diseases, making the regulation of iron metabolism essential for tissue repair. In our analysis of degenerated intervertebral disc tissue, we observe a positive correlation between the concentration of extracellular iron ions (ex-iron) and the severity of ferroptosis in intervertebral disc degeneration (IVDD). Hence, inspired by magnets attracting metals, we combine polyether F127 diacrylate (FDA) with tannin (TA) to construct a magnetically attracting hydrogel (FDA-TA). This hydrogel demonstrates the capability to adsorb ex-iron and remodel the iron metabolism of cells. Furthermore, it exhibits good toughness and self-healing properties. Notably, it can activate the PI3K-AKT pathway to inhibit nuclear receptor coactivator 4-mediated ferritinophagy under ex-iron enrichment conditions. The curative effect and related mechanism are further confirmed in vivo. Consequently, on the basis of the pathological mechanism, a targeted hydrogel is designed to reshape iron metabolism, offering insights for tissue repair.
Figures



References
-
- Liu C., Shen Y., Cavdar O., Huang J., Fang H., Angiotensin II-induced vascular endothelial cells ferroptosis via P53-ALOX12 signal axis. Clin.Exp. Hypertens. 45, 2180019 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

