Epinephrine promotes breast cancer metastasis through a ubiquitin-specific peptidase 22-mediated lipolysis circuit
- PMID: 39151008
- PMCID: PMC11328899
- DOI: 10.1126/sciadv.ado1533
Epinephrine promotes breast cancer metastasis through a ubiquitin-specific peptidase 22-mediated lipolysis circuit
Abstract
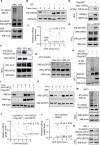
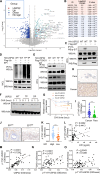
Chronic stress-induced epinephrine (EPI) accelerates breast cancer progression and metastasis, but the molecular mechanisms remain unclear. Herein, we found a strong positive correlation between circulating EPI levels and the tumoral expression of ubiquitin-specific peptidase 22 (USP22) in patients with breast cancer. USP22 facilitated EPI-induced breast cancer progression and metastasis by enhancing adipose triglyceride lipase (ATGL)-mediated lipolysis. Targeted USP22 deletion decreased ATGL expression and lipolysis, subsequently inhibiting EPI-mediated breast cancer lung metastasis. USP22 acts as a bona fide deubiquitinase for the Atgl gene transcription factor FOXO1, and EPI architects a lipolysis signaling pathway to stabilize USP22 through AKT-mediated phosphorylation. Notably, USP22 phosphorylation levels are positively associated with EPI and with downstream pathways involving both FOXO1 and ATGL in breast cancers. Pharmacological USP22 inhibition synergized with β-blockers in treating preclinical xenograft breast cancer models. This study reveals a molecular pathway behind EPI's tumor-promoting effects and provides a strong rationale for combining USP22 inhibition with β-blockers to treat aggressive breast cancer.
Figures





References
-
- Weigelt B., Peterse J. L., van’t Veer L. J., Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 5, 591–602 (2005). - PubMed
-
- Spiegel D., Bloom J. R., Kraemer H. C., Gottheil E., Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 2, 888–891 (1989). - PubMed
-
- Borgi M., Collacchi B., Ortona E., Cirulli F., Stress and coping in women with breast cancer: Unravelling the mechanisms to improve resilience. Neurosci. Biobehav. Rev. 119, 406–421 (2020). - PubMed
-
- Chang A., Botteri E., Gillis R. D., Lofling L., Le C. P., Ziegler A. I., Chung N. C., Rowe M. C., Fabb S. A., Hartley B. J., Nowell C. J., Kurozumi S., Gandini S., Munzone E., Montagna E., Eikelis N., Phillips S. E., Honda C., Masuda K., Katayama A., Oyama T., Cole S. W., Lambert G. W., Walker A. K., Sloan E. K., Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 15, eadf1147 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

