Optimal systemic treatment and real-world clinical application of ctDNA in patients with metastatic HER2-mutant lung cancer
- PMID: 39151324
- PMCID: PMC12327952
- DOI: 10.1016/j.ejca.2024.114257
Optimal systemic treatment and real-world clinical application of ctDNA in patients with metastatic HER2-mutant lung cancer
Abstract
Introduction: No definitive answers currently exist regarding optimal first-line therapy for HER2-mutant NSCLC. Access to rapid tissue sequencing is a major barrier to precision drug development in the first-line setting. ctDNA analysis has the potential to overcome these obstacles and guide treatment.
Methods: We retrospectively analyzed patients with metastatic HER2-mutant NSCLC who underwent prospective clinical ctDNA sequencing and received systemic therapy at Memorial Sloan Kettering Cancer Center (MSK) from January 2016 to September 2022. HER2 mutations were identified by next-generation sequencing through MSK-IMPACT, MSK-ACCESS or Resolution ctDx LungTM assay. Primary endpoints were time to the next treatment (TTNT) and overall survival (OS).
Results: Sixty-three patients were included in the primary analysis. Chemoimmunotherapy (33/63, 52.4 %) was the predominant first-line treatment with a median TTNT of 5.1 months (95 %CI 4.1 - 6.1) whereas 55.0 % (22/40) of patients who received second-line T-DXd obtained a median TTNT of 9.2 m (95 % CI, 0-22.2). Plasma ctDNA was tested before first-line therapy in 40 patients with a median OS of 28.0 months (95 % CI 21-34), in whom 31 patients (78.0 %) had detectable ctDNA. HER2 mutations were detected on ctDNA with a median turnaround time of 13 days, occasionally co-occurred with EGFR and MET alterations and were tracked longitudinally correlating with treatment response. Patients with detectable baseline ctDNA had significantly shorter OS (hazard ratio (HR), 5.25; 95 % CI, 1.2-23.9; p = 0.019).
Conclusion: Chemoimmunotherapy remains a major treatment option for metastatic HER2-mutant NSCLC. ctDNA can rapidly detect HER2 and co-mutations, and it has the potential to guide and monitor optimal first-line therapy. As a negative prognostic biomarker, detectable ctDNA at baseline would need to be taken into account for patient selection in future studies.
Keywords: CtDNA; HER2 mutation; Immunotherapy; NSCLC; Targeted therapy.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Bob T. Li has served as an uncompensated advisor and consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, and Lilly. He has received research grants to his institution from Amgen, AstraZeneca, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, Jiangsu Hengrui Pharmaceuticals, Lilly, Nuvalent, and Revolution Medicines. He has received academic travel support from Amgen. He is an inventor on three institutional patents at MSK (US62/685,057, US62/514,661, US63/424,813) and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. Alexander Drilon declared as follows: Honoraria: 14ner/Elevation Oncology, Amgen, Abbvie, ArcherDX, AstraZeneca, Beigene, BergenBio, Blueprint Medicines, Bristol Myers Squibb, Chugai Pharmaceutical, EcoR1, EMD Serono, Entos, Exelixis, Helsinn, Hengrui Therapeutics, Ignyta/Genentech/Roche, Janssen, Loxo/Bayer/Lilly, Merus, Monopteros, MonteRosa, Novartis, Nuvalent, Pfizer, Prelude, Regeneron, Repare RX, Springer Healthcare, Takeda/Ariad/Millenium, Treeline Bio, TP Therapeutics, Tyra Biosciences, Verastem. Advisory Boards: Bayer, MonteRosa, Abbvie, EcoR1 Capital, LLC, Amgen, Helsinn, Novartis, Lilly, AnHeart Therapeutics. Consulting: MonteRosa, Innocare, Boundless Bio, Treeline Bio, Nuvalent, 14ner/Elevation Oncology, Entos, Prelude. Associated Research Paid to Institution: Foundatin Medicine, GlaxoSmithKlein, Teva, Taiho, PharmaMar. Equity: mBrace. Copyright: Selpercatinib-Osimertinib (filed/pending). Royalties: Wolters Kluwer; Other (Food/Beverage): Merck, Puma, Merus, Boehringer Ingelheim; CME Honoraria: Answers in CME, Applied Pharmaceutical Science, Inc, AXIS, Clinical Care Options, Doc Congress, EPG Health, Harborside Nexus, I3 Health, Imedex, Liberum, Medendi, Medscape, Med Learning, MedTalks, MJH Life Sciences, MORE Health, Ology, OncLive, Paradigm, Peerview Institute, PeerVoice, Physicians Education, Projects in Knowledge, Resources, Remedica Ltd, Research to Practice, RV More, Targeted Oncology, TouchIME, WebMD. Sarat Chandarlapaty has received institutional grant/funding from Daiichi-Sankyo, AstraZeneca, and Lilly, Shares/Ownership interests in Odyssey Biosciences, Effector Therapeutics, and Totus Medicines, and consultation/Ad board/Honoraria from AstraZeneca, Daiichi-Sankyo, Novartis, Neogenomics, Nuvalent, Blueprint, SAGA Diagnostics, and Effector Therapeutics. Jiannan Guo is a former employee of Resolution Bioscience. Eric Gagne, Kavita Garg and Frederick Baehner are employees of Exact Sciences. All remaining authors have declared no conflict of interest.
Figures
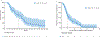
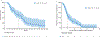

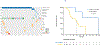


References
-
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nature medicine. 2021;27:1345–56. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. - PubMed
-
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY). 2004;304:1497–500. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. - PubMed
-
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

