Imaging macular carotenoids and their related proteins in the human retina with confocal resonance Raman and fluorescence microscopy
- PMID: 39151780
- PMCID: PMC11412777
- DOI: 10.1016/j.exer.2024.110043
Imaging macular carotenoids and their related proteins in the human retina with confocal resonance Raman and fluorescence microscopy
Abstract
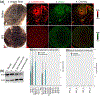
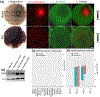
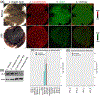
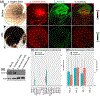
Lutein and zeaxanthin are highly concentrated at the central region of the human retina, forming a distinct yellow spot known as the macula lutea. The delivery and retention of the macular pigment carotenoids in the macula lutea involves many proteins, but their exact roles remain incompletely understood. In our study, we examined the distribution of the twelve known macular carotenoid-related proteins within the human macula and the underlying retinal pigment epithelium (RPE) using both fluorescence and Raman modes on our confocal resonance Raman microscope. Additionally, we assessed protein and gene expression through Western blot analysis and a single-cell RNA sequencing database. Our findings revealed that GSTP1, BCO2, and Aster-B exhibited distribution patterns similar to the macular carotenoids, with higher expression levels within the macular region compared to the periphery, while SR-BI and ABCA1 did not exhibit specific distribution patterns within the macula or RPE. Interestingly, LIPC, SR-BI's partner, accumulated specifically in the sub-foveal RPE. All three of these carotenoid transport proteins were found to be highly expressed in the RPE. These results offer valuable insights into the roles these proteins play in the formation of the macula lutea.
Keywords: BCO2; Carotenoid; Fluorescence; GSTP1; Macular pigment; Raman.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest.
Figures
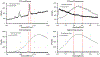








References
-
- Age-Related Eye Disease Study 2 Research, G., 2013. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 309, 2005–2015. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

