Click Chemistry Enables [89Zr]Zr-DOTA Radioimmunoconjugation for Theranostic 89Zr-immunoPET
- PMID: 39151917
- PMCID: PMC11583970
- DOI: 10.1021/acs.bioconjchem.4c00274
Click Chemistry Enables [89Zr]Zr-DOTA Radioimmunoconjugation for Theranostic 89Zr-immunoPET
Abstract
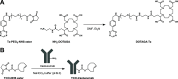
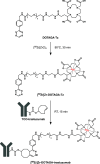
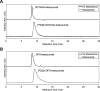
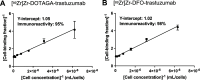
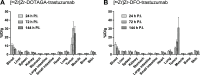
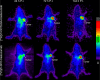
There have been predictions that the use of the macrocyclic chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) in zirconium-89 (89Zr) immuno-positron emission tomography (89Zr-immunoPET) could enhance the in vivo stability of 89Zr radioimmunoconjugates. However, conjugating [89Zr]Zr-DOTA to a monoclonal antibody (mAb) remains a challenge as the heat treatment required for [89Zr]Zr-DOTA chelation can lead to thermal denaturation of the mAb moieties. We developed a method for synthesizing [89Zr]Zr-DOTA-mAb based on a tetrazine (Tz)-conjugated bifunctional DOTA derivative 2,2',2″-(10-(1-(4-(1,2,4,5-tetrazin-3-yl)phenyl)-3,21,26-trioxo-6,9,12,15,18-pentaoxa-29-carboxy-2,22,25-triazanonacosane-29-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (DOTAGA-Tz) and the inverse electron-demand Diels-Alder (IEDDA) click chemistry reaction where trans-cyclooctene-modified mAbs are conjugated to [89Zr]Zr-DOTAGA without being exposed to heat. The stability of IEDDA-derived [89Zr]Zr-DOTAGA-trastuzumab was confirmed by in vitro, ex vivo, and in vivo testing and comparative analysis against the conventional deferoxamine (DFO) counterpart [89Zr]Zr-DFO-trastuzumab. The in vivo immunoPET imaging using [89Zr]Zr-DOTAGA-trastuzumab clearly visualized human epidermal growth factor receptor 2-positive malignancies in murine xenograft models. Greater tumor contrast was observed from [89Zr]Zr-DOTAGA-trastuzumab at a 72-h delayed scan compared with [89Zr]Zr-DFO-trastuzumab. These findings suggest that our IEDDA ligation approach can be an effective means of synthesizing [89Zr]Zr-DOTA-mAb and can enhance the theranostic potential of 89Zr-immunoPET in DOTA-mediated radioimmunotherapy.
Copyright © 2024 The Authors. Published by American Chemical Society
Conflict of interest statement
The authors declare the following competing financial interest(s): Ryota Imura and Hiroyuki Ida are employees of JFE Engineering Corporation, a commercial supplier of 89Zr.
Figures

Similar articles
-
Imaging with [89Zr]Zr-DFO-SC16.56 anti-DLL3 antibody in patients with high-grade neuroendocrine tumours of the lung and prostate: a phase 1/2, first-in-human trial.Lancet Oncol. 2024 Aug;25(8):1015-1024. doi: 10.1016/S1470-2045(24)00249-3. Epub 2024 Jun 28. Lancet Oncol. 2024. PMID: 38950555 Free PMC article. Clinical Trial.
-
Evaluation and selection of a lead diabody for interferon-γ PET imaging.Nucl Med Biol. 2022 Nov-Dec;114-115:162-167. doi: 10.1016/j.nucmedbio.2022.06.001. Epub 2022 Jun 17. Nucl Med Biol. 2022. PMID: 35753939 Free PMC article.
-
177Lu-Anti-CD25 Antibody for Interleukin-2 Receptor-α-Targeted Radioimmunotherapy of SUDHL1 Lymphomas in Mice.Mol Pharm. 2025 Jul 7;22(7):3702-3714. doi: 10.1021/acs.molpharmaceut.4c01410. Epub 2025 May 23. Mol Pharm. 2025. PMID: 40408541
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
Cited by
-
89Zr-Radiolabelling of p-NCS-Bz-DFO-Anti-HER2 Affibody Immunoconjugate: Characterization and Assessment of In Vitro Potential in HER2-Positive Breast Cancer Imaging.Pharmaceutics. 2025 Jun 4;17(6):739. doi: 10.3390/pharmaceutics17060739. Pharmaceutics. 2025. PMID: 40574051 Free PMC article.
References
-
- Le Loirec C.; Champion C. Track structure simulation for positron emitters of medical interest. Part I: The case of the allowed decay isotopes. Nucl. Instrum. Methods Phys. Res., Sect. A 2007, 582, 644–653. 10.1016/j.nima.2007.08.159. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

