NR4A ablation improves mitochondrial fitness for long persistence in human CAR-T cells against solid tumors
- PMID: 39151930
- PMCID: PMC11331892
- DOI: 10.1136/jitc-2023-008665
NR4A ablation improves mitochondrial fitness for long persistence in human CAR-T cells against solid tumors
Abstract
Background: Antitumor effect of chimeric antigen receptor (CAR)-T cells against solid tumors is limited due to various factors, such as low infiltration rate, poor expansion capacity, and exhaustion of T cells within the tumor. NR4A transcription factors have been shown to play important roles in T-cell exhaustion in mice. However, the precise contribution of each NR4a factor to human T-cell differentiation remains to be clarified.
Methods: In this study, we deleted NR4A family factors, NR4A1, NR4A2, and NR4A3, in human CAR-T cells recognizing human epidermal growth factor receptor type 2 (HER2) by using the CRISPR/Cas9 system. We induced T-cell exhaustion in these cells in vitro through repeated co-culturing of CAR-T cells with Her2+A549 lung adenocarcinoma cells and evaluated cell surface markers such as memory and exhaustion phenotypes, proliferative capacity, cytokine production and metabolic activity. We validated the antitumor toxicity of NR4A1/2/3 triple knockout (TKO) CAR-T cells in vivo by transferring CAR-T cells into A549 tumor-bearing immunodeficient mice.
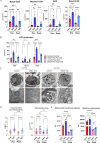
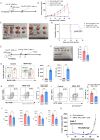
Results: Human NR4A-TKO CAR-T cells were resistant against exhaustion induced by repeated antigen stimulation in vitro, and maintained higher tumor-killing activity both in vitro and in vivo compared with control CAR-T cells. A comparison of the effectiveness of NR4A single, double, and TKOs demonstrated that triple KO was the most effective in avoiding exhaustion. Furthermore, a strong enhancement of antitumor effects by NR4A TKO was also observed in T cells from various donors including aged persons. Mechanistically, NR4A TKO CAR-T cells showed enhanced mitochondrial oxidative phosphorylation, therefore could persist for longer periods within the tumors.
Conclusions: NR4A factors regulate CAR-T cell persistence and stemness through mitochondrial gene expression, therefore NR4A is a highly promising target for the generation of superior CAR-T cells against solid tumors.
Keywords: Adoptive cell therapy - ACT; Chimeric antigen receptor - CAR; Lung Cancer; Solid tumor; Stem cell.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Similar articles
-
NR4A transcription factors limit CAR T cell function in solid tumours.Nature. 2019 Mar;567(7749):530-534. doi: 10.1038/s41586-019-0985-x. Epub 2019 Feb 27. Nature. 2019. PMID: 30814732 Free PMC article.
-
CD70 CAR-T cells empowered by TS-2021 through ex vivo transduction show potent antitumor efficacy against glioblastoma.J Exp Clin Cancer Res. 2025 Jun 5;44(1):173. doi: 10.1186/s13046-025-03431-6. J Exp Clin Cancer Res. 2025. PMID: 40474210 Free PMC article.
-
BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion.Sci Transl Med. 2022 Nov 9;14(670):eabn7336. doi: 10.1126/scitranslmed.abn7336. Epub 2022 Nov 9. Sci Transl Med. 2022. PMID: 36350986 Free PMC article.
-
The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms.Curr Drug Targets. 2015;16(1):38-46. doi: 10.2174/1389450115666141120112818. Curr Drug Targets. 2015. PMID: 25410408 Review.
-
Natural products and synthetic analogs as selective orphan nuclear receptor 4A (NR4A) modulators.Histol Histopathol. 2024 May;39(5):543-556. doi: 10.14670/HH-18-689. Epub 2023 Dec 13. Histol Histopathol. 2024. PMID: 38116863 Free PMC article. Review.
Cited by
-
Advances in strategies to improve the immunotherapeutic efficacy of chimeric antigen receptor-T cell therapy for lymphoma.Cancer Biol Med. 2025 Apr 15;22(4):301-21. doi: 10.20892/j.issn.2095-3941.2024.0538. Cancer Biol Med. 2025. PMID: 40231980 Free PMC article. Review.
-
Innovative approaches to CAR-T cell therapy: the role of lipid metabolism pathways.J Transl Med. 2025 Jun 17;23(1):670. doi: 10.1186/s12967-025-06718-6. J Transl Med. 2025. PMID: 40528186 Free PMC article. Review.
-
Targeting the nuclear orphan receptor NR4A1: a key target in lung cancer progression and therapeutic resistance.Front Oncol. 2025 Jul 1;15:1566598. doi: 10.3389/fonc.2025.1566598. eCollection 2025. Front Oncol. 2025. PMID: 40666103 Free PMC article. Review.
-
Enhancing the potency of CAR-T cells against solid tumors through transcription factor engineering.JCI Insight. 2025 Jul 22;10(14):e193048. doi: 10.1172/jci.insight.193048. eCollection 2025 Jul 22. JCI Insight. 2025. PMID: 40693464 Free PMC article. Review.
-
Constitutive IL-7 signaling promotes CAR-NK cell survival in the solid tumor microenvironment but impairs tumor control.J Immunother Cancer. 2025 Jul 23;13(7):e010672. doi: 10.1136/jitc-2024-010672. J Immunother Cancer. 2025. PMID: 40707132 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
