Experience Dependence of Alpha Rhythms and Neural Dynamics in the Mouse Visual Cortex
- PMID: 39151954
- PMCID: PMC11411595
- DOI: 10.1523/JNEUROSCI.2011-22.2024
Experience Dependence of Alpha Rhythms and Neural Dynamics in the Mouse Visual Cortex
Abstract
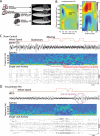
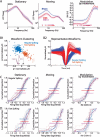
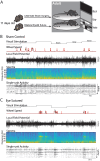
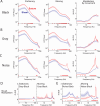
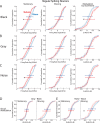
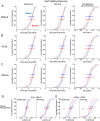
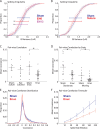
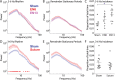
The role of experience in the development and maintenance of emergent network properties such as cortical oscillations and states is poorly understood. To define how early-life experience affects cortical dynamics in the visual cortex of adult, head-fixed mice, we examined the effects of two forms of blindness initiated before eye opening and continuing through recording: (1) bilateral loss of retinal input (enucleation) and (2) degradation of visual input (eyelid suture). Neither form of deprivation fundamentally altered the state-dependent regulation of firing rates or local field potentials. However, each deprivation caused unique changes in network behavior. Laminar analysis revealed two different generative mechanisms for low-frequency synchronization: one prevalent during movement and the other during quiet wakefulness. The former was absent in enucleated mice, suggesting a mouse homolog of human alpha oscillations. In addition, neurons in enucleated animals were less correlated and fired more regularly, but no change in mean firing rate. Eyelid suture decreased firing rates during quiet wakefulness, but not during movement, with no effect on neural correlations or regularity. Sutured animals showed a broadband increase in depth EEG power and an increased occurrence, but reduced central frequency, of narrowband gamma oscillations. The complementary-rather than additive-effects of lid suture and enucleation suggest that the development of emergent network properties does not require vision but is plastic to modified input. Our results suggest a complex interaction of internal set points and experience determines mature cortical activity, with low-frequency synchronization being particularly susceptible to early deprivation.
Keywords: alpha; arousal; blindness; gamma; oscillations; vision.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Experience-Dependent Development of Feature-Selective Synchronization in the Primary Visual Cortex.J Neurosci. 2018 Sep 5;38(36):7852-7869. doi: 10.1523/JNEUROSCI.0027-18.2018. Epub 2018 Jul 31. J Neurosci. 2018. PMID: 30064994 Free PMC article.
-
Regional Specificity of GABAergic Regulation of Cross-Modal Plasticity in Mouse Visual Cortex after Unilateral Enucleation.J Neurosci. 2015 Aug 12;35(32):11174-89. doi: 10.1523/JNEUROSCI.3808-14.2015. J Neurosci. 2015. PMID: 26269628 Free PMC article.
-
Effects of monocular enucleation, tetrodotoxin, and lid suture on cytochrome-oxidase reactivity in supragranular puffs of adult macaque striate cortex.Vis Neurosci. 1990 Mar;4(3):185-204. doi: 10.1017/s0952523800003345. Vis Neurosci. 1990. PMID: 1964078
-
Human brain plasticity: evidence from sensory deprivation and altered language experience.Prog Brain Res. 2002;138:177-88. doi: 10.1016/S0079-6123(02)38078-6. Prog Brain Res. 2002. PMID: 12432770 Review.
-
Phase correlation among rhythms present at different frequencies: spectral methods, application to microelectrode recordings from visual cortex and functional implications.Int J Psychophysiol. 1997 Jun;26(1-3):171-89. doi: 10.1016/s0167-8760(97)00763-0. Int J Psychophysiol. 1997. PMID: 9203002 Review.
References
-
- Adrian ED, Matthew BHC (1934) The Berger rhythm: potential changes from the occipital lobes in man. Brrain 57:355–385. 10.1093/brain/57.4.355 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
