Genome-wide variation study and inter-tissue communication analysis unveil regulatory mechanisms of egg-laying performance in chickens
- PMID: 39152103
- PMCID: PMC11329784
- DOI: 10.1038/s41467-024-50809-9
Genome-wide variation study and inter-tissue communication analysis unveil regulatory mechanisms of egg-laying performance in chickens
Abstract
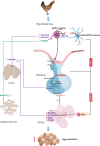
Egg-laying performance is of great economic importance in poultry, but the underlying genetic mechanisms are still elusive. In this work, we conduct a multi-omics and multi-tissue integrative study in hens with distinct egg production, to detect the hub candidate genes and construct hub molecular networks contributing to egg-laying phenotypic differences. We identifiy three hub candidate genes as egg-laying facilitators: TFPI2, which promotes the GnRH secretion in hypothalamic neuron cells; CAMK2D, which promotes the FSHβ and LHβ secretion in pituitary cells; and OSTN, which promotes granulosa cell proliferation and the synthesis of sex steroid hormones. We reveal key endocrine factors involving egg production by inter-tissue crosstalk analysis, and demonstrate that both a hepatokine, APOA4, and an adipokine, ANGPTL2, could increase egg production by inter-tissue communication with hypothalamic-pituitary-ovarian axis. Together, These results reveal the molecular mechanisms of multi-tissue coordinative regulation of chicken egg-laying performance and provide key insights to avian reproductive regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

