Intra-tumoral and peripheral blood TIGIT and PD-1 as immune biomarkers in nodular lymphocyte predominant Hodgkin lymphoma
- PMID: 39152767
- PMCID: PMC11469944
- DOI: 10.1002/ajh.27459
Intra-tumoral and peripheral blood TIGIT and PD-1 as immune biomarkers in nodular lymphocyte predominant Hodgkin lymphoma
Abstract
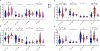
In classical Hodgkin lymphoma (cHL), responsiveness to immune-checkpoint blockade (ICB) is associated with specific tumor microenvironment (TME) and peripheral blood features. The role of ICB in nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is not established. To gain insights into its potential in NLPHL, we compared TME and peripheral blood signatures between HLs using an integrative multiomic analysis. A discovery/validation approach in 121 NLPHL and 114 cHL patients highlighted >2-fold enrichment in programmed cell death-1 (PD-1) and T-cell Ig and ITIM domain (TIGIT) gene expression for NLPHL versus cHL. Multiplex imaging showed marked increase in intra-tumoral protein expression of PD-1+ (and/or TIGIT+) CD4+ T-cells and PD-1+CD8+ T-cells in NLPHL compared to cHL. This included T-cells that rosetted with lymphocyte predominant (LP) and Hodgkin Reed-Sternberg (HRS) cells. In NLPHL, intra-tumoral PD-1+CD4+ T-cells frequently expressed TCF-1, a marker of heightened T-cell response to ICB. The peripheral blood signatures between HLs were also distinct, with higher levels of PD-1+TIGIT+ in TH1, TH2, and regulatory CD4+ T-cells in NLPHL versus cHL. Circulating PD-1+CD4+ had high levels of TCF-1. Notably, in both lymphomas, highly expanded populations of clonal TIGIT+PD-1+CD4+ and TIGIT+PD-1+CD8+ T-cells in the blood were also present in the TME, indicating that immune-checkpoint expressing T-cells circulated between intra-tumoral and blood compartments. In in vitro assays, ICB was capable of reducing rosette formation around LP and HRS cells, suggesting that disruption of rosetting may be a mechanism of action of ICB in HL. Overall, results indicate that further evaluation of ICB is warranted in NLPHL.
© 2024 The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
E.A.H: Research funding to institution; Bristol Myers Squibb, Merck KgA, Astra Zeneca, Roche, Advisory board: Roche*, Antigene*, Bristol Myers Squibb, Astra Zeneca, Novartis*, Merck Sharpe Dohme*, Gilead*, Beigene* (*paid to institution) Speakers fees: Roche*, Astra Zeneca*, Abbvie*, Janssen, Regeneron, (*paid to institution) Consultancy: Regeneron, Bristol Myers Squibb, Specialized therapeutics. D.T: research funding from Roche, Janssen and Beigene, and honoraria from CSL, Antengene, Roche, Beigene, Amgen, and EUSA Pharma. M.K.G.: honoraria from Novartis, Gilead. F.V. receives research funding from Caribou, Allogene, Geron corporation and received in the last 3 years honoraria from Oakstone Medical Publishing, i3Health, Elsevier, America Registry of Pathology, Congressionally Directed Medical Research Program, Society of Hematology Oncology and National Research Foundation of Singapore (28th Competitive Research Program Whitepapers). E.B: consultancy Abbvie, Astellas, Gilead, IQVIA, MSD, Novartis and Bastion Brands, Research funding MSD. MRG: research funding from Sanofi, Kite/Gilead, Abbvie and Allogene; consulting for Abbvie, Allogene and Bristol Myers Squibb; honoraria from BMS, Daiichi Sankyo and DAVA Oncology; and stock ownership of KDAc Therapeutics.
Figures


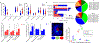
References
-
- Hartmann S, Eichenauer DA, Plutschow A, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood. 2013;122(26):4246–4252; quiz 4292. - PubMed
-
- Savage KJ, Mottok A, Fanale M. Nodular lymphocyte-predominant Hodgkin lymphoma. Semin Hematol. 2016;53(3):190–202. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
