Modular Chip-Based nanoSFC-MS for Ultrafast Separations
- PMID: 39152902
- PMCID: PMC11359387
- DOI: 10.1021/acs.analchem.4c01958
Modular Chip-Based nanoSFC-MS for Ultrafast Separations
Abstract
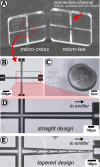
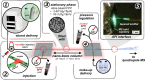
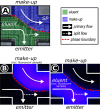
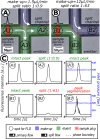
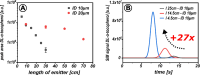
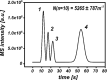
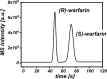
This study presents the development of a miniaturized device for supercritical fluid chromatography coupled with mass spectrometry. The chip-based, modular nanoSFC approach utilizes a particle-packed nanobore column embedded between two monolithically structured glass chips. A microtee in the pre-column section ensures picoliter sample loads onto the column, while a microcross chip structure fluidically controls the column backpressure. The restrictive emitter and the minimal post-column volume of 16 nL prevent mobile phase decompression and analyte dilution, maintaining chromatographic integrity during transfer to the atmospheric pressure MS interface. This facilitates high-speed chiral separations in less than 80 s with high reproducibility.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Welch C. J. React. Chem. Eng. 2019, 4, 1895.10.1039/C9RE00234K. - DOI
LinkOut - more resources
Full Text Sources

