Silver-based bimetallic nanozyme fabrics with peroxidase-mimic activity for urinary glucose detection
- PMID: 39153105
- PMCID: PMC11511708
- DOI: 10.1007/s00216-024-05483-7
Silver-based bimetallic nanozyme fabrics with peroxidase-mimic activity for urinary glucose detection
Abstract
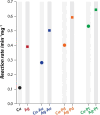
The enhanced catalytic properties of bimetallic nanoparticles have been extensively investigated. In this study, bimetallic Ag-M (M = Au, Pt, or Pd) cotton fabrics were fabricated using a combination of electroless deposition and galvanic replacement reactions, and improvement in their peroxidase-mimicking catalytic activity compared to that of the parent Ag fabric was studied. The Ag-Pt bimetallic nanozyme fabric, which showed the highest catalytic activity and ability to simultaneously generate hydroxyl (•OH) and superoxide (O2•-) radicals, was assessed as a urine glucose sensor. This nanozyme fabric sensor could directly detect urinary glucose in the pathophysiologically relevant high millimolar range without requiring sample predilution. The sensor could achieve performance on par with that of the current clinical gold standard assay. These features of the Ag-Pt nanozyme sensor, particularly its ability to avoid interference effects from complex urinary matrices, position it as a viable candidate for point-of-care urinary glucose monitoring.
Keywords: Atypical peroxidase; Bimetallic nanoparticles; Colorimetric; Functional fabrics; Nanozyme; Urinalysis.
© 2024. The Author(s).
Conflict of interest statement
The authors have no financial, non-financial, or proprietary interests in any material discussed in this article. Professor Vipul Bansal is a guest editor of ABC but was not involved in the peer review of this paper.
Figures




References
-
- Toshima N, Yonezawa T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem. 1998;22(11):1179–201. 10.1039/A805753B.
-
- Bansal V, O’Mullane AP, Bhargava SK. Galvanic replacement mediated synthesis of hollow Pt nanocatalysts: significance of residual Ag for the H2 evolution reaction. Electrochem Commun. 2009;11(8):1639–42. 10.1016/j.elecom.2009.06.018.
-
- Harpeness R, Gedanken A. Microwave synthesis of core− shell gold/palladium bimetallic nanoparticles. Langmuir. 2004;20(8):3431–4. 10.1021/la035978z. - PubMed
-
- Chen S, Jenkins SV, Tao J, Zhu Y, Chen J. Anisotropic seeded growth of Cu–M (M= Au, Pt, or Pd) bimetallic nanorods with tunable optical and catalytic properties. J Phys Chem C. 2013;117(17):8924–32. 10.1021/jp4013653.
-
- Bansal V, Jani H, Du Plessis J, Coloe PJ, Bhargava SK. Galvanic replacement reaction on metal films: a one-step approach to create nanoporous surfaces for catalysis. Adv Mater. 2008;20(4):717–23. 10.1002/adma.200701297.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

