Intravenous ferric carboxymaltose versus oral ferrous sulphate for the treatment of moderate to severe postpartum anaemia in Nigerian women (IVON-PP): protocol for an open-label randomised controlled type 1 hybrid effectiveness-implementation trial
- PMID: 39153791
- PMCID: PMC11331826
- DOI: 10.1136/bmjopen-2024-086553
Intravenous ferric carboxymaltose versus oral ferrous sulphate for the treatment of moderate to severe postpartum anaemia in Nigerian women (IVON-PP): protocol for an open-label randomised controlled type 1 hybrid effectiveness-implementation trial
Abstract
Introduction: Postpartum anaemia is often caused by iron deficiency with onset during the antepartum period and can be exacerbated by excessive blood loss at birth. Its prevalence is estimated as 50-80% in low-income and middle-income countries. It poses adverse consequences on the mother and negatively impacts her ability to care for her newborn. Prompt treatment of postpartum anaemia is thus important. Adherence to oral iron is reportedly low in Nigeria due to its side effects and forgetfulness by the mothers. Intravenous iron such as ferric carboxymaltose, given as a single dose, might help overcome adherence issues, but investigation in a high-quality randomised control trial in Nigeria is first required while evaluation of challenges around its implementation is also warranted.
Objective: To determine the clinical effectiveness, tolerability and safety, of using intravenous ferric carboxymaltose (intervention) vs oral ferrous sulphate (control) for treating moderate to severe iron deficiency anaemia in postpartum women and to evaluate implementation of ferric carboxymaltose in treating postpartum anaemia in Nigeria.
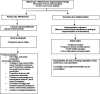
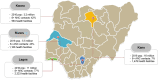
Methods and analysis: This study is an open-label randomised controlled trial with a concurrent implementation study. It is a hybrid type 1 effectiveness-implementation design conducted in four states across Northern and Southern Nigeria. A total of 1400 eligible and consenting women with postpartum moderate to severe anaemia (haemoglobin concentration <100 g/L) will be randomised to intravenous ferric carboxymaltose; a single dose at 20 mg/kg to a maximum of 1000 mg infusion administered at enrolment (intervention) or oral ferrous sulphate; 200 mg (65 mg elemental iron) two times per day from enrolment until 6 weeks postpartum (control). The primary outcome, proportion of participants who are anaemic (Hb <110 g/L) at 6 weeks postpartum will be analysed by intention-to-treat. Haemoglobin concentration, full blood count, serum iron, serum ferritin, transferrin saturation and total iron binding capacity will be measured at specific intervals. Implementation outcomes such as acceptability and feasibility of using ferric carboxymaltose for postpartum anaemia treatment in Nigeria will be assessed.
Ethics and dissemination: This study is approved by the ethics committee of the teaching hospitals, Ministry of Health of the four states as required, National Health Research Ethics Committee and the drug regulatory agency, National Agency for Food and Drug Administration and Control (NAFDAC). Findings of this research will be presented at conferences and will be published in international peer-reviewed journals and shared with stakeholders within and outside Nigeria.
Trial registration number: International standard randomised controlled trial number: ISRCTN51426226.
Keywords: anaemia; depression & mood disorders; postpartum women; quality of life.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Eboreime E, Banke-Thomas A, Obi-Jeff C, et al. A continuous quality improvement strategy to strengthen screening practices and facilitate the routine use of intravenous iron for treating anaemia in pregnant and postpartum women in Nigeria: A study protocol. Impl Sci Commun. 2023;4:22. doi: 10.1186/s43058-023-00400-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
