Plasma proteome profiling in giant cell arteritis
- PMID: 39153834
- PMCID: PMC11563890
- DOI: 10.1136/ard-2024-225868
Plasma proteome profiling in giant cell arteritis
Abstract
Objectives: This study aimed to identify plasma proteomic signatures that differentiate active and inactive giant cell arteritis (GCA) from non-disease controls. By comprehensively profiling the plasma proteome of both patients with GCA and controls, we aimed to identify plasma proteins that (1) distinguish patients from controls and (2) associate with disease activity in GCA.
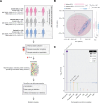
Methods: Plasma samples were obtained from 30 patients with GCA in a multi-institutional, prospective longitudinal study: one captured during active disease and another while in clinical remission. Samples from 30 age-matched/sex-matched/race-matched non-disease controls were also collected. A high-throughput, aptamer-based proteomics assay, which examines over 7000 protein features, was used to generate plasma proteome profiles from study participants.
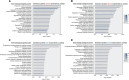
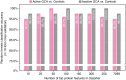
Results: After adjusting for potential confounders, we identified 537 proteins differentially abundant between active GCA and controls, and 781 between inactive GCA and controls. These proteins suggest distinct immune responses, metabolic pathways and potentially novel physiological processes involved in each disease state. Additionally, we found 16 proteins associated with disease activity in patients with active GCA. Random forest models trained on the plasma proteome profiles accurately differentiated active and inactive GCA groups from controls (95.0% and 98.3% in 10-fold cross-validation, respectively). However, plasma proteins alone provided limited ability to distinguish between active and inactive disease states within the same patients.
Conclusions: This comprehensive analysis of the plasma proteome in GCA suggests that blood protein signatures integrated with machine learning hold promise for discovering multiplex biomarkers for GCA.
Keywords: Giant Cell Arteritis; Machine Learning; Vasculitis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

