Pulmonary fibrosis may begin in infancy: from childhood to adult interstitial lung disease
- PMID: 39153860
- PMCID: PMC11671978
- DOI: 10.1136/thorax-2024-221772
Pulmonary fibrosis may begin in infancy: from childhood to adult interstitial lung disease
Abstract
Background: Childhood interstitial lung disease (chILD) encompasses a group of rare heterogeneous respiratory conditions associated with significant morbidity and mortality. Reports suggest that many patients diagnosed with chILD continue to have potentially progressive or fibrosing disease into adulthood. Over the last decade, the spectrum of conditions within chILD has widened substantially, with the discovery of novel entities through advanced genetic testing. However, most evidence is often limited to small case series, with reports disseminated across an array of subspecialty, clinical and molecular journals. In particular, the frequency, management and outcome of paediatric pulmonary fibrosis is not well characterised, unlike in adults, where clear diagnosis and treatment guidelines are available.
Methods and results: This review assesses the current understanding of pulmonary fibrosis in chILD. Based on registry data, we have provisionally estimated the occurrence of fibrosis in various manifestations of chILD, with 47 different potentially fibrotic chILD entities identified. Published evidence for fibrosis in the spectrum of chILD entities is assessed, and current and future issues in management of pulmonary fibrosis in childhood, continuing into adulthood, are considered.
Conclusions: There is a need for improved knowledge of chILD among pulmonologists to optimise the transition of care from paediatric to adult facilities. Updated evidence-based guidelines are needed that incorporate recommendations for the diagnosis and management of immune-mediated disorders, as well as chILD in older children approaching adulthood.
Keywords: Connective tissue disease associated lung disease; Drug induced Lung Disease; Interstitial Fibrosis; Paediatric Lung Disaese; Paediatric interstitial lung disease; Rare lung diseases; Surfactant protein; Systemic disease and lungs.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MG reports grants from Boehringer Ingelheim for a register analysis regarding fibrosis, paid to his institution; consulting fees from Boehringer Ingelheim and Roche; speaker fees from Boehringer Ingelheim; payment for participation on an adjudication and on an advisory board from Boehringer Ingelheim; and payment for a leadership role in a board society from Vertex. GK has been a faculty member of a paediatric bronchoscopy course, unrelated to the submitted work. MC, RE and DW have nothing to disclose. GD reports grants from NIH and CZI Atlas, paid to their institution; consulting fees from Boehringer Ingelheim as a consulting pathologist on the InPedILD trial, paid to their institution; and support for attending meetings and/or travel from NIH, paid to their institution. NN reports consulting fees from AstraZeneca, unrelated to the submitted work; support for attending meetings and/or travel for COST action CA16125 and CIG16125; and serving as head of a clinical research collaboration for chILD (unpaid). NS reports grants for participation on an advisory board, an expense allowance, payment for lectures and support for attending meetings and/or travel from Boehringer Ingelheim; serving as president elect of the German Society for Pediatric Pneumology and serving as vice chair of Kinderlungenregister. JW reports personal fees and non-financial support from Boehringer Ingelheim and personal fees from Parexel/Calyx. LRY reports grants from the NIH and University of Pennsylvania, consultancy fees from Roche, Sanofi and Boehringer Ingelheim, and honoraria from NYU Langone Health. RD reports two grants from Boehringer Ingelheim; consulting fees from Boehringer Ingelheim and Roche; licensed patents and stocks for Now Vitals, of which she is a founder, EvoEndoscopy, of which she is a founder and Earable, of which she is a founder; personal fees and non-financial support from Boehringer Ingelheim; and personal fees and other from Earable Inc, Now Vitals and EvoEndoscopy.
Figures


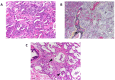
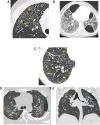
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
