Modeling gastric intestinal metaplasia in 3D organoids using nitrosoguanidine
- PMID: 39153963
- PMCID: PMC11744189
- DOI: 10.1093/jmcb/mjae030
Modeling gastric intestinal metaplasia in 3D organoids using nitrosoguanidine
Abstract
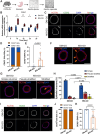
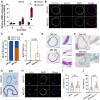
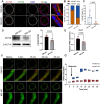
Gastric intestinal metaplasia (GIM) represents a precancerous stage characterized by morphological and pathophysiological changes in the gastric mucosa, where gastric epithelial cells transform into a phenotype resembling that of intestinal cells. Previous studies have demonstrated that the intragastric administration of N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) induces both gastric carcinoma and intestinal metaplasia in mice. Here, we show that MNNG induces GIM in three-dimensional (3D) mouse organoids. Our histological analyses reveal that MNNG-induced gastric organoids undergo classical morphological alterations, exhibiting a distinct up-regulation of CDX2 and MUC2, along with a down-regulation of ATP4B and MUC6. Importantly, metaplastic cells observed in MNNG-treated organoids originate from MIST1+ cells, indicating their gastric chief cell lineage. Functional analyses show that activation of the RAS signaling pathway drives MNNG-induced metaplasia in 3D organoids, mirroring the characteristics observed in human GIM. Consequently, modeling intestinal metaplasia using 3D organoids offers valuable insights into the molecular mechanisms and spatiotemporal dynamics of the gastric epithelial lineage during the development of intestinal metaplasia within the gastric mucosa. We conclude that the MNNG-induced metaplasia model utilizing 3D organoids provides a robust platform for developing preventive and therapeutic strategies to mitigate the risk of gastric cancer before precancerous lesions occur.
Keywords: MNNG; gastric organoids; intestinal metaplasia; precancerous lesion; trans-differentiation.
© The Author(s) (2024). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures



References
-
- Barros R., Freund J.N., David L. et al. (2012). Gastric intestinal metaplasia revisited: function and regulation of CDX2. Trends Mol. Med. 18, 555–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

