Granulosa cell insight: unraveling the potential of menstrual blood-derived stem cells and their exosomes on mitochondrial mechanisms in polycystic ovary syndrome (PCOS)
- PMID: 39153978
- PMCID: PMC11330151
- DOI: 10.1186/s13048-024-01484-3
Granulosa cell insight: unraveling the potential of menstrual blood-derived stem cells and their exosomes on mitochondrial mechanisms in polycystic ovary syndrome (PCOS)
Abstract
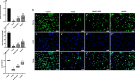
Background: Polycystic ovary syndrome (PCOS) presents a significant challenge in women's reproductive health, characterized by disrupted folliculogenesis and ovulatory dysfunction. Central to PCOS pathogenesis are granulosa cells, whose dysfunction contributes to aberrant steroid hormone production and oxidative stress. Mitochondrial dysfunction emerges as a key player, influencing cellular energetics, oxidative stress, and steroidogenesis. This study investigates the therapeutic potential of menstrual blood-derived stem cells (MenSCs) and their exosomes in mitigating mitochondrial dysfunction and oxidative stress in PCOS granulosa cells.
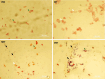
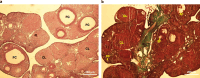
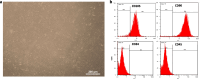
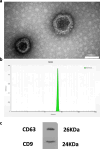
Methods: Using a rat model of PCOS induced by letrozole, granulosa cells were harvested and cultured. MenSCs and their exosomes were employed to assess their effects on mitochondrial biogenesis, oxidative stress, and estrogen production in PCOS granulosa cells.
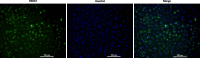
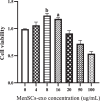
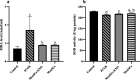
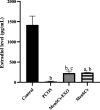
Results: Results showed diminished mitochondrial biogenesis and increased oxidative stress in PCOS granulosa cells, alongside reduced estrogen production. Treatment with MenSCs and their exosomes demonstrated significant improvements in mitochondrial biogenesis, oxidative stress levels, and estrogen production in PCOS granulosa cells. Further analysis showed MenSCs' superior efficacy over exosomes, attributed to their sustained secretion of bioactive factors. Mechanistically, MenSCs and exosomes activated pathways related to mitochondrial biogenesis and antioxidative defense, highlighting their therapeutic potential for PCOS.
Conclusions: This study offers insights into granulosa cells mitochondria's role in PCOS pathogenesis and proposes MenSCs and exosomes as a potential strategy for mitigating mitochondrial dysfunction and oxidative stress in PCOS. Further research is needed to understand underlying mechanisms and validate clinical efficacy, presenting promising avenues for addressing PCOS complexity.
Keywords: Exosomes; Granulosa cells; Menstrual blood-derived stem cells (MenSCs); Mitochondrial biogenesis; Polycystic ovary syndrome (PCOS).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

