Nodal Response and Survival After Neoadjuvant Endocrine Therapy in Hormone Receptor-Positive Breast Cancer: 20-Year Experience from a Single Institution
- PMID: 39154153
- PMCID: PMC11560637
- DOI: 10.1245/s10434-024-16059-1
Nodal Response and Survival After Neoadjuvant Endocrine Therapy in Hormone Receptor-Positive Breast Cancer: 20-Year Experience from a Single Institution
Abstract
Introduction: Axillary response to neoadjuvant endocrine therapy (NET) for the treatment of hormone receptor-positive breast cancer (HR+ BC) is not well-described. This study was designed to characterize nodal response after NET.
Methods: Patients receiving NET followed by curative intent surgery at a comprehensive cancer center from 1998 to 2022 in a prospectively collected registry were included. Patients with distant metastasis were excluded. Primary outcome was nodal pathologic complete response (pCR). Downstaging was defined as post-NET decrease in category.
Results: We included 123 patients; the majority were cT2 (n = 59) or cT3 (n = 35), and cN0 (n = 81). Median age was 70.0 years (interquartile range 62.1-76.0). Forty-two patients (34.1%) were clinically node-positive. After NET, 73 (59.8%) underwent breast-conserving surgery. All patients underwent sentinel lymph node biopsy, and 12 (9.8%) underwent completion axillary lymph node dissection. In-breast downstaging was achieved in 51 (41.5%) patients, 1 (0.8%) had breast pCR, and 14 (11.4%) had breast upstaging. Axillary downstaging was achieved in 10 (23.8%), 6 patients (14.3%) had nodal pCR, and 14 (33.3%) had axillary upstaging. At 10-year follow-up, local recurrence was 1% and distant recurrence was 14%, while disease-free survival was 82%. After adjusting for demographic and clinical factors, age was the only characteristic associated with mortality (hazard ratio 1.07, 95% confidence interval 1.01-1.13).
Conclusions: In HR+ BC treated with NET, long-term disease-free survival is good, although nodal pCR is uncommon for cN+ patients. Future studies are needed to elucidate optimal neoadjuvant systemic therapy and to delineate oncologically safe strategies to deescalate axillary management for residual microscopic disease.
Keywords: Axilla; Breast cancer; Endocrine therapy; Neoadjuvant; Node.
© 2024. Society of Surgical Oncology.
Figures
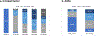


References
-
- Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, Regan MM, Spears PA, Sudheendra PK, Symmans WF, Yung RL, Harvey BE, Hershman DL. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol. 2021. May 1;39(13):1485–1505. doi: 10.1200/JCO.20.03399. Epub 2021 Jan 28. - DOI - PMC - PubMed
-
- Madigan LI, Dinh P, Graham JD. Neoadjuvant endocrine therapy in locally advanced estrogen or progesterone receptor-positive breast cancer: determining the optimal endocrine agent and treatment duration in postmenopausal women-a literature review and proposed guidelines. Breast Cancer Res. 2020;22(1):77. Published 2020 Jul 20. doi:10.1186/s13058-020-01314-6 - DOI - PMC - PubMed
-
- Barchiesi G, Mazzotta M, Krasniqi E, Pizzuti L, Marinelli D, Capomolla E, Sergi D, Amodio A, Natoli C, Gamucci T, Vizza E, Marchetti P, Botti C, Sanguineti G, Ciliberto G, Barba M, Vici P. Neoadjuvant Endocrine Therapy in Breast Cancer: Current Knowledge and Future Perspectives. Int J Mol Sci. 2020. May 16;21(10):3528. doi: 10.3390/ijms21103528. - DOI - PMC - PubMed
-
- Hunt KK, Suman VJ, Wingate HF, et al. Local-Regional Recurrence After Neoadjuvant Endocrine Therapy: Data from ACOSOG Z1031 (Alliance), a Randomized Phase 2 Neoadjuvant Comparison Between Letrozole, Anastrozole, and Exemestane for Postmenopausal Women with Estrogen Receptor-Positive Clinical Stage 2 or 3 Breast Cancer. Ann Surg Oncol. 2023;30(4):2111–2118. doi:10.1245/s10434-022-12972-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

