Small RNA-Seq and real time rt-qPCR reveal islet miRNA released under stress conditions
- PMID: 39154325
- PMCID: PMC11332650
- DOI: 10.1080/19382014.2024.2392343
Small RNA-Seq and real time rt-qPCR reveal islet miRNA released under stress conditions
Abstract
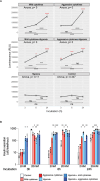
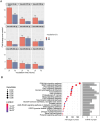
Replacement of beta cells through transplantation is a potential therapeutic approach for individuals with pancreas removal or poorly controllable type 1 diabetes. However, stress and death of beta cells pose significant challenges. Circulating miRNA has emerged as potential biomarkers reflecting early beta cell stress and death, allowing for timely intervention. The aim of this study was to identify miRNAs as potential biomarkers for beta cell health. Literature review combined with small RNA sequencing was employed to select islet-enriched miRNA. The release of those miRNA was assessed by RT-qPCR in vivo, using a streptozotocin induced diabetes mouse model and in vitro, through mouse and human islets exposed to varying degrees of hypoxic and cytokine stressors. Utilizing the streptozotocin induced model, we identified 18 miRNAs out of 39 candidate islet-enriched miRNA to be released upon islet stress in vivo. In vitro analysis of culture supernatants from cytokine and/or hypoxia stressed islets identified the release of 45 miRNAs from mouse and 8 miRNAs from human islets. Investigation into the biological pathways targeted by the cytokine- and/or hypoxia-induced miRNA suggested the involvement of MAPK and PI3K-Akt signaling pathways in both mouse and human islets. We have identified miRNAs associated with beta cell health and stress. The findings allowed us to propose a panel of 47 islet-related human miRNA that is potentially valuable for application in clinical contexts of beta cell transplantation and presymptomatic early-stage type 1 diabetes.
Keywords: Biomarker; T1D; islet beta cell stress; miRNA; transplantation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. doi:10.1517/03009734000000059. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
