Non-canonical autophosphorylation of RIPK1 drives timely pyroptosis to control Yersinia infection
- PMID: 39154339
- PMCID: PMC11465231
- DOI: 10.1016/j.celrep.2024.114641
Non-canonical autophosphorylation of RIPK1 drives timely pyroptosis to control Yersinia infection
Abstract
Caspase-8-dependent pyroptosis has been shown to mediate host protection from Yersinia infection. For this mode of cell death, the kinase activity of receptor-interacting protein kinase 1 (RIPK1) is required, but the autophosphorylation sites required to drive caspase-8 activation have not been determined. Here, we show that non-canonical autophosphorylation of RIPK1 at threonine 169 (T169) is necessary for caspase-8-mediated pyroptosis. Mice with alanine in the T169 position are highly susceptible to Yersinia dissemination. Mechanistically, the delayed formation of a complex containing RIPK1, ZBP1, Fas-associated protein with death domain (FADD), and caspase-8 abrogates caspase-8 maturation in T169A mice and leads to the eventual activation of RIPK3-dependent necroptosis in vivo; however, this is insufficient to protect the host, suggesting that timely pyroptosis during early response is specifically required to control infection. These results position RIPK1 T169 phosphorylation as a driver of pyroptotic cell death critical for host defense.
Keywords: CP: Immunology; CP: Microbiology; RIPK1; Yersinia pseudotuberculosis; YopJ; autophosphorylation; caspase-8; macrophages; necroptosis; pyroptosis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
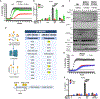


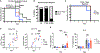
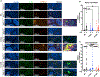
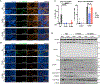
References
-
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, et al. (2014). Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl. Acad. Sci. 111, 7385–7390. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

