Blood inflammation relates to neuroinflammation and survival in frontotemporal lobar degeneration
- PMID: 39155063
- PMCID: PMC7617268
- DOI: 10.1093/brain/awae269
Blood inflammation relates to neuroinflammation and survival in frontotemporal lobar degeneration
Abstract
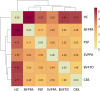
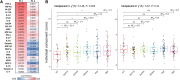
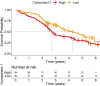
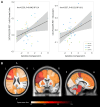
Neuroinflammation is an important pathogenic mechanism in many neurodegenerative diseases, including those caused by frontotemporal lobar degeneration. Post-mortem and in vivo imaging studies have shown brain inflammation early in these conditions, proportional to symptom severity and rate of progression. However, evidence for corresponding blood markers of inflammation and their relationships to central inflammation and clinical outcome are limited. There is a pressing need for such scalable, accessible and mechanistically relevant blood markers because these will reduce the time, risk and costs of experimental medicine trials. We therefore assessed inflammatory patterns of serum cytokines from 214 patients with clinical syndromes associated with frontotemporal lobar degeneration in comparison to healthy controls, including their correlation with brain regional microglial activation and disease progression. Serum assays used the MesoScale Discovery V-Plex-Human Cytokine 36 plex panel plus five additional cytokine assays. A subgroup of patients underwent 11C-PK11195 mitochondrial translocator protein PET imaging, as an index of microglial activation. A principal component analysis was used to reduce the dimensionality of cytokine data, excluding cytokines that were undetectable in >50% of participants. Frequentist and Bayesian analyses were performed on the principal components to compare each patient cohort with controls and test for associations with central inflammation, neurodegeneration-related plasma markers and survival. The first component identified by the principal component analysis (explaining 21.5% variance) was strongly loaded by pro-inflammatory cytokines, including TNF-α, TNF-R1, M-CSF, IL-17A, IL-12, IP-10 and IL-6. Individual scores of the component showed significant differences between each patient cohort and controls. The degree to which a patient expressed this peripheral inflammatory profile at baseline was correlated negatively with survival (higher inflammation, shorter survival), even when correcting for baseline clinical severity. Higher pro-inflammatory profile scores were associated with higher microglial activation in frontal and brainstem regions, as quantified with 11C-PK11195 mitochondrial translocator protein PET. A permutation-based canonical correlation analysis confirmed the association between the same cytokine-derived pattern and central inflammation across brain regions in a fully data-based manner. This data-driven approach identified a pro-inflammatory profile across the frontotemporal lobar degeneration clinical spectrum, which is associated with central neuroinflammation and worse clinical outcome. Blood-based markers of inflammation could increase the scalability and access to neuroinflammatory assessment of people with dementia, to facilitate clinical trials and experimental medicine studies.
Keywords: PET; blood markers; frontotemporal lobar degeneration; inflammation; survival.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors have no conflicts of interest to report related to this work. Unrelated to this work, M.M. has acted as a consultant for Astex Pharmaceuticals. J.T.O. has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai and Novo Nordisk and has acted as a consultant for Biogen and Roche and has received research support from Alliance Medical and Merck. J.B.R. is a non-remunerated trustee of the Guarantors of Brain and Darwin College. He provides consultancy unrelated to the current work to Asceneuron, Astronautx, Astex, Alector, Curasen, CumulusNeuro, Prevail, Wave, SVHealth and has research grants from AstraZeneca, Janssen, GSK and Lilly as industry partners in the Dementias Platform UK. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk and Roche and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Figures
References
MeSH terms
Substances
Grants and funding
- National Institute for Health Research
- Distinguished Professor at the Swedish Research Council
- Department of Health and Social Care
- Creative Commons Attribution 4.0 International License
- #FO2022-0270/Hjärnfonden
- #ALFGBG-71320/Swedish State Support for Clinical Research
- WT_/Wellcome Trust/United Kingdom
- #2023-00356/Swedish Research Council
- 101053962/European Union's Horizon Europe research
- #ADSF-21-831376-C/ALZ/Alzheimer's Association/United States
- Addenbrookes Charitable Trust
- #201809-2016862/Alzheimer Drug Discovery Foundation
- G101149/Guarantors of Brain
- GRANTS002/Progressive Supranuclear Palsy Association
- NIHR203312/Cambridge Biomedical Research Centre
- 220258/WT_/Wellcome Trust/United Kingdom
- P30 AG072980/AG/NIA NIH HHS/United States
- MC_UU_00030/14/MRC_/Medical Research Council/United Kingdom
- JPND2021-00694/European Union Joint Programme-Neurodegenerative Disease Research
- Erling-Persson Family Foundation
- UKDRI-1003/UK Dementia Research Institute at UCL
- Bluefield Project
- 860197/Marie Skłodowska-Curie
- AD Strategic Fund
- (ARUK-RADF2021A-010/Race Against Dementia Alzheimer's Research UK
- European Union's Horizon 2020 research and innovation programme
- Stiftelsen för Gamla Tjänarinnor
- Cure Alzheimer's Fund
- 602/ALZS_/Alzheimer's Society/United Kingdom
- the Olav Thon Foundation
- Wallenberg Scholar
- Dementias Platform UK
- (RG95450/Cambridge University Centre for Parkinson-Plus
LinkOut - more resources
Full Text Sources
Research Materials

