Purification and biochemical characterization of methanobactin biosynthetic enzymes
- PMID: 39155110
- PMCID: PMC11622243
- DOI: 10.1016/bs.mie.2024.06.011
Purification and biochemical characterization of methanobactin biosynthetic enzymes
Abstract
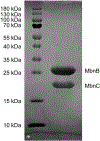
Methanobactin (Mbn) is a ribosomally synthesized and post-translationally modified peptide (RiPP) natural product that binds Cu(I) with high affinity. The copper-chelating thioamide/oxazolone groups in Mbn are installed on the precursor peptide MbnA by the core enzyme complex, MbnBC, which includes the multinuclear non-heme iron-dependent oxidase (MNIO) MbnB and its RiPP recognition element-containing partner protein MbnC. For the extensively characterized Mbn biosynthetic gene cluster (BGC) from the methanotroph Methylosinus trichosporium OB3b, the tailoring aminotransferase MbnN further modifies MbnA after leader sequence cleavage by an unknown mechanism. Here we detail methods to express and purify M. trichosporium OB3b MbnBC and MbnN along with protocols for assessing MbnA modification by MbnBC and MbnN aminotransferase activity. In addition, we describe crystallization and structure determination of MbnBC. These procedures can be adapted for other MNIOs and partner proteins encoded in Mbn and Mbn-like BGCs. Furthermore, these methods provide a first step toward in vitro biosynthesis of Mbns and related natural products as potential therapeutics.
Keywords: Aminotransferase; Chalkophore; Copper homeostasis; DUF692; Heterologous expression; MNIO; Methanobactin; Methanotroph; Multinuclear iron enzyme; RiPP natural product.
Copyright © 2024. Published by Elsevier Inc.
Figures


Similar articles
-
An Aminotransferase Is Responsible for the Deamination of the N-Terminal Leucine and Required for Formation of Oxazolone Ring A in Methanobactin of Methylosinus trichosporium OB3b.Appl Environ Microbiol. 2016 Dec 15;83(1):e02619-16. doi: 10.1128/AEM.02619-16. Print 2017 Jan 1. Appl Environ Microbiol. 2016. PMID: 27795312 Free PMC article.
-
Methanobactins: Structures, Biosynthesis, and Microbial Diversity.Annu Rev Microbiol. 2024 Nov;78(1):383-401. doi: 10.1146/annurev-micro-041522-092911. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 39121541 Free PMC article. Review.
-
The enzymology of oxazolone and thioamide synthesis in methanobactin.Methods Enzymol. 2021;656:341-373. doi: 10.1016/bs.mie.2021.04.008. Epub 2021 May 7. Methods Enzymol. 2021. PMID: 34325792
-
The biosynthesis of methanobactin.Science. 2018 Mar 23;359(6382):1411-1416. doi: 10.1126/science.aap9437. Science. 2018. PMID: 29567715 Free PMC article.
-
Chalkophores.Annu Rev Biochem. 2018 Jun 20;87:645-676. doi: 10.1146/annurev-biochem-062917-012300. Epub 2018 Apr 18. Annu Rev Biochem. 2018. PMID: 29668305 Free PMC article. Review.
References
-
- Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, … van der Donk WA (2013). Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Natural Product Reports, 30, 108–160. 10.1039/C2NP20085F. - DOI - PMC - PubMed
-
- Behling LA, Hartsel SC, Lewis DE, Dispirito AA, Choi DW, Masterson LR, … Gallagher WH (2008). NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings. Journal of the American Chemical Society, 130, 12604–12605. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

