Pharmacokinetics and pharmacodynamics of PTC518, an oral huntingtin lowering splicing modifier: A first-in-human study
- PMID: 39155237
- PMCID: PMC11602954
- DOI: 10.1111/bcp.16202
Pharmacokinetics and pharmacodynamics of PTC518, an oral huntingtin lowering splicing modifier: A first-in-human study
Abstract
Aims: PTC518 is an orally administered, centrally and peripherally distributed huntingtin (HTT) pre-mRNA splicing modifier being developed for the treatment of Huntington's disease (HD) for which there is a high unmet medical need as there are currently no approved disease-modifying treatments. This first-in-human study investigated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of PTC518 in healthy volunteers.
Methods: This phase 1, single-centre, randomized study in 77 healthy male and female volunteers evaluated the safety and tolerability and PK of PTC518 following single ascending doses and multiple ascending doses, PD as assessed by HTT mRNA and HTT protein levels after single and multiple doses, and food effects.
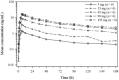
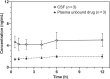
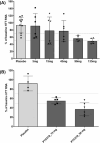
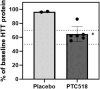
Results: PTC518 demonstrated a favourable safety profile. The majority of treatment-emergent adverse events were mild and transient. PTC518 Tmax was reached at 6-7 h and the terminal T1/2 was 54.0-75.3 h following a single oral dose. Exposure increased with dose though less than dose proportionally. The PTC518 concentrations in cerebrospinal fluid were approximately 2.6-fold higher than the unbound free-drug concentrations in plasma. A significant dose-dependent reduction of up to approximately 60% in HTT mRNA and a significant dose-dependent, time-dependent and sustained reduction in HTT protein levels of up to 35% were observed after PTC518 treatment.
Conclusions: PTC518 was well tolerated, and proof of mechanism of this novel splicing modifier was demonstrated by the dose-dependent decrease in systemic HTT mRNA and HTT protein levels. Results from this first-in-human study support further studies in patients with HD and demonstrate the potential for PTC518 as a breakthrough treatment for HD.
Keywords: genetic diseases; neuroscience; pharmacokinetics–pharmacodynamics; phase 1.
© 2024 PTC Therapeutics, Inc. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Lan Gao, Anuradha Bhattacharyya, Brian Beers, Diksha Kaushik, Amy‐Lee Bredlau, Allan Kristensen, Richard Grant, Lee Golden and Ronald Kong are employees and stock owners of PTC Therapeutics.
Khalid Abd‐Elaziz is an employee of the University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Figures

References
-
- Caron NS, Wright GEB, Hayden MR. Huntington disease. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews® [internet]. University of Washington, Seattle; 1998. Oct 23 [updated 2020 Jun 11]:1993‐2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

