Basal forebrain-lateral habenula inputs and control of impulsive behavior
- PMID: 39155312
- PMCID: PMC11480124
- DOI: 10.1038/s41386-024-01963-7
Basal forebrain-lateral habenula inputs and control of impulsive behavior
Abstract
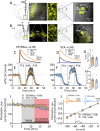
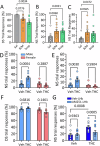
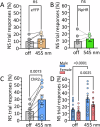
Deficits in impulse control are observed in several neurocognitive disorders, including attention deficit hyperactivity (ADHD), substance use disorders (SUDs), and those following traumatic brain injury (TBI). Understanding brain circuits and mechanisms contributing to impulsive behavior may aid in identifying therapeutic interventions. We previously reported that intact lateral habenula (LHb) function is necessary to limit impulsivity defined by impaired response inhibition in rats. Here, we examine the involvement of a synaptic input to the LHb on response inhibition using cellular, circuit, and behavioral approaches. Retrograde fluorogold tracing identified basal forebrain (BF) inputs to LHb, primarily arising from ventral pallidum and nucleus accumbens shell (VP/NAcs). Glutamic acid decarboxylase and cannabinoid CB1 receptor (CB1R) mRNAs colocalized with fluorogold, suggesting a cannabinoid modulated GABAergic pathway. Optogenetic activation of these axons strongly inhibited LHb neuron action potentials and GABA release was tonically suppressed by an endogenous cannabinoid in vitro. Behavioral experiments showed that response inhibition during signaled reward omission was impaired when VP/NAcs inputs to LHb were optogenetically stimulated, whereas inhibition of this pathway did not alter LHb control of impulsivity. Systemic injection with the psychotropic phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC), also increased impulsivity in male, and not female rats, and this was blocked by LHb CB1R antagonism. However, as optogenetic VP/NAcs pathway inhibition did not alter impulse control, we conclude that the pro-impulsive effects of Δ9-THC likely do not occur via inhibition of this afferent. These results identify an inhibitory LHb afferent that is controlled by CB1Rs that can regulate impulsive behavior.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Vonder Haar C, Lam FC, Adams WK, Riparip LK, Kaur S, Muthukrishna M, et al. Frontal traumatic brain injury in rats causes long-lasting impairments in impulse control that are differentially sensitive to pharmacotherapeutics and associated with chronic neuroinflammation. ACS Chem Neurosci. 2016;7:1531–42. - PMC - PubMed
-
- Beauchaine TP, Zisner AR, Sauder CL. Trait impulsivity and the externalizing spectrum. Annu Rev Clin Psychol. 2017;13:343–68. - PubMed
-
- Dougherty DM, Mathias CW, Marsh DM, Moeller FG, Swann AC. Suicidal behaviors and drug abuse: impulsivity and its assessment. Drug Alcohol Depend. 2004;76:S93–s105. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

