Control of Intestinal Stemness and Cell Lineage by Histone Variant H2A.Z Isoforms
- PMID: 39155414
- PMCID: PMC11529411
- DOI: 10.1080/10985549.2024.2387720
Control of Intestinal Stemness and Cell Lineage by Histone Variant H2A.Z Isoforms
Abstract
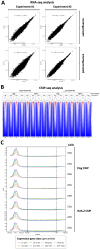
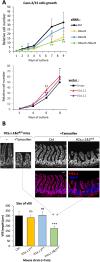
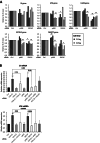
The histone variant H2A.Z plays important functions in the regulation of gene expression. In mammals, it is encoded by two genes, giving rise to two highly related isoforms named H2A.Z.1 and H2A.Z.2, which can have similar or antagonistic functions depending on the promoter. Knowledge of the physiopathological consequences of such functions emerges, but how the balance between these isoforms regulates tissue homeostasis is not fully understood. Here, we investigated the relative role of H2A.Z isoforms in intestinal epithelial homeostasis. Through genome-wide analysis of H2A.Z genomic localization in differentiating Caco-2 cells, we uncovered an enrichment of H2A.Z isoforms on the bodies of genes which are induced during enterocyte differentiation, stressing the potential importance of H2A.Z isoforms dynamics in this process. Through a combination of in vitro and in vivo experiments, we further demonstrated the two isoforms cooperate for stem and progenitor cells proliferation, as well as for secretory lineage differentiation. However, we found that they antagonistically regulate enterocyte differentiation, with H2A.Z.1 preventing terminal differentiation and H2A.Z.2 favoring it. Altogether, these data indicate that H2A.Z isoforms are critical regulators of intestine homeostasis and may provide a paradigm of how the balance between two isoforms of the same chromatin structural protein can control physiopathological processes.
Keywords: H2A.Z histone variant; differentiation; gene expression; intestinal epithelial homeostasis; proliferation.
Conflict of interest statement
There are no relevant financial or non-financial competing interests to report.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
