The impact of analytical treatment interruptions and trial interventions on time to viral re-suppression in people living with HIV restarting ART in cure-related clinical studies: a systematic review and meta-analysis
- PMID: 39155436
- PMCID: PMC11330850
- DOI: 10.1002/jia2.26349
The impact of analytical treatment interruptions and trial interventions on time to viral re-suppression in people living with HIV restarting ART in cure-related clinical studies: a systematic review and meta-analysis
Abstract
Introduction: To assess the effectiveness of novel HIV curative strategies, "cure" trials require periods of closely monitored antiretroviral therapy (ART) analytical treatment interruptions (ATIs). We performed a systematic review and meta-analysis to identify the impact of ATI with or without novel therapeutics in cure-related studies on the time to viral re-suppression following ART restart.
Methods: Medline, Embase and Web of Science databases were searched for human studies involving ATIs from 1 January 2015 till 22 April 2024. The primary outcome was time to first viral re-suppression (plasma HIV viral load [VL] <50 copies/ml) stratified by receipt of interventional drug with ATI (IA) or ATI-only groups. Random-effects proportional meta-analysis and multivariable Cox proportional hazards analysis were performed using R.
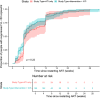
Results: Of 1073 studies screened, 13 were included that met the inclusion criteria with VL data available after restarting ART (n = 213 participants). There was no difference between time to viral suppression in IA or ATI-only cohorts (p = 0.22). For 87% of participants, viral suppression within 12 weeks of ART restart was achieved, and all eventually had at least one VL <50 copies/ml during follow-up. After adjusting for covariables, while participants in the IA cohort were associated with less rapid suppression (adjusted hazard ratio [aHR] 0.61, 95% CI 0.40-0.94, p = 0.026), other factors include greater log VL at ART restart (aHR 0.56, 95% CI 0.46-0.68, p<0.001), duration since HIV diagnosis (aHR 0.93, 95% CI 0.89-0.96) and longer intervals between HIV VL monitoring (aHR 0.66, 95% CI 0.59-0.74, p<0.001). However, the use of integrase inhibitors was associated with more rapid viral suppression (aHR 1.74, 95% CI 1.16-2.59).
Discussion: When designing studies involving ATIs, information on time to viral re-suppression after restarting ART is important to share with participants, and should be regularly monitored and reported, to assess the impact and safety of specific trial interventions in ATI studies.
Conclusions: The majority of participants achieved viral suppression after restarting ART in ATI studies. ART regimens containing integrase inhibitors and frequent VL monitoring should be offered for people restarting ART after ATI studies to ensure rapid re-suppression.
Keywords: ATI; HIV; HIV cure; antiretroviral therapy; treatment interruption; viral suppression.
© 2024 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
MJL is supported by the UK Medical Research Council Clinical Research Training Fellowship (MR/W024454/1), and has received speakers’ fees, conference from Viiv Healthcare, Gilead Sciences, and consultancy fees from Thriva Limited, external to the submitted work. EF has received grants and support for attending meetings from Viiv Healthcare and Gilead. BM has received stock options and consulting fees from AELIX Therapeutics SL, consulting fees from AbbviE, and speakers’ fees from Gilead, Janssen and Viiv Healthcare, outside the submitted work; and also participates on the data safety monitoring board for Leyden Labs. JLR is funded by the following NIH grants (NIH U19AI117950, NIH UM1AI164570) and has received stock options, royalties or licenses from Kite Pharma, BlueWhale Bio, and consulting fees from Scietemex Consulting, all external to the submitted work. JDG is supported by the Lundbeck Foundation (R381–2021–1405). All other authors have declared no conflicts of interests.
Figures

References
-
- Lau JSY, Smith MZ, Lewin SR, McMahon JH. Clinical trials of antiretroviral treatment interruption in HIV‐infected individuals. AIDS. 2019;33(5):773–791. - PubMed
-
- Stecher M, Claßen A, Klein F, Lehmann C, Gruell H, Platten M, et al. Systematic review and meta‐analysis of treatment interruptions in human immunodeficiency virus (HIV) type 1‐infected patients receiving antiretroviral therapy: implications for future HIV cure trials. Clin Infect Dis. 2020;70(7):1406–1417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

