POLE2 silencing inhibits the progression of colorectal carcinoma cells via wnt signaling axis
- PMID: 39155507
- PMCID: PMC11340749
- DOI: 10.1080/15384047.2024.2392339
POLE2 silencing inhibits the progression of colorectal carcinoma cells via wnt signaling axis
Abstract
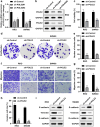
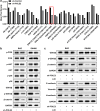
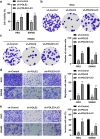
Colorectal cancer (CRC) is one of the most common malignant carcinoma worldwide. DNA polymerase epsilon 2, accessory subunit (POLE2) participates in DNA replication, repair, and cell cycle control, but its association with CRC development remains unclear. In the present study, the differentially expressed genes (DEGs) in CRC were screened from bioinformatics analysis based on GEO database. RT-qPCR was used to assess mRNA expression. CCK-8 and colony formation assays were applied for the evaluation of cell proliferation. Wound healing and transwell assays were used to detect cell migration and invasion. Protein levels were determined by Western blotting assay. We found that POLE2 was highly expressed in CRC tissues and cell lines. Inhibition of POLE2 suppressed the proliferation, migration and invasion of CRC cells. Mechanistically, Wnt/β-catenin signaling pathway was inactivated by inhibition of POLE2. Activation of Wnt/β-catenin pathway can reverse the function of POLE2 knockdown on CRC cells. In vivo studies demonstrated that POLE2 silencing could notably inhibit the growth of tumors, which was consistent with the results in vitro. In conclusion, we found POLE2 as a novel oncogene in CRC, providing a potential therapeutic or diagnostic target in CRC.
Keywords: Colorectal cancer; POLE2; Wnt/β-catenin; invasion; proliferation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
POLE2 knockdown suppresses lymphoma progression via downregulating Wnt/β-catenin signaling pathway.Mol Cell Biochem. 2024 Mar;479(3):487-497. doi: 10.1007/s11010-023-04738-8. Epub 2023 Apr 25. Mol Cell Biochem. 2024. PMID: 37097331
-
Tumour suppressor ABCA8 inhibits malignant progression of colorectal cancer via Wnt/β-catenin pathway.Dig Liver Dis. 2024 May;56(5):880-893. doi: 10.1016/j.dld.2023.10.026. Epub 2023 Nov 13. Dig Liver Dis. 2024. PMID: 37968146
-
Forkhead Box S1 mediates epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway to regulate colorectal cancer progression.J Transl Med. 2022 Jul 21;20(1):327. doi: 10.1186/s12967-022-03525-1. J Transl Med. 2022. PMID: 35864528 Free PMC article.
-
Histone Demethylase JMJD2D Interacts With β-Catenin to Induce Transcription and Activate Colorectal Cancer Cell Proliferation and Tumor Growth in Mice.Gastroenterology. 2019 Mar;156(4):1112-1126. doi: 10.1053/j.gastro.2018.11.036. Epub 2018 Nov 23. Gastroenterology. 2019. PMID: 30472235
-
TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer.J Exp Clin Cancer Res. 2020 Nov 5;39(1):232. doi: 10.1186/s13046-020-01690-z. J Exp Clin Cancer Res. 2020. PMID: 33153498 Free PMC article.
Cited by
-
[NIP7 upregulates the expression of ubiquitin-conjugating enzyme E2 C to promote tumor growth in anaplastic thyroid cancer].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 May 25;54(3):372-381. doi: 10.3724/zdxbyxb-2024-0708. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40461291 Free PMC article. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
