Fully human monoclonal antibodies against Ebola virus possess complete protection in a hamster model
- PMID: 39155772
- PMCID: PMC11348817
- DOI: 10.1080/22221751.2024.2392651
Fully human monoclonal antibodies against Ebola virus possess complete protection in a hamster model
Abstract
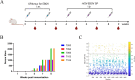
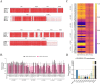
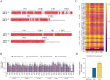
Ebola disease is a lethal viral hemorrhagic fever caused by ebolaviruses within the Filoviridae family with mortality rates of up to 90%. Monoclonal antibody (mAb) based therapies have shown great potential for the treatment of EVD. However, the potential emerging ebolavirus isolates and the negative effect of decoy protein on the therapeutic efficacy of antibodies highlight the necessity of developing novel antibodies to counter the threat of Ebola. Here, 11 fully human mAbs were isolated from transgenic mice immunized with GP protein and recombinant vesicular stomatitis virus-bearing GP (rVSV-EBOV GP). These mAbs were divided into five groups according to their germline genes and exhibited differential binding activities and neutralization capabilities. In particular, mAbs 8G6, 2A4, and 5H4 were cross-reactive and bound at least three ebolavirus glycoproteins. mAb 4C1 not only exhibited neutralizing activity but no cross-reaction with sGP. mAb 7D8 exhibited the strongest neutralizing capacity. Further analysis on the critical residues for the bindings of 4C1 and 8G6 to GPs was conducted using antibodies complementarity-determining regions (CDRs) alanine scanning. It has been shown that light chain CDR3 played a crucial role in binding and neutralization and that any mutation in CDRs could not improve the binding of 4C1 to sGP. Importantly, mAbs 7D8, 8G6, and 4C1 provided complete protections against EBOV infection in a hamster lethal challenge model when administered 12 h post-infection. These results support mAbs 7D8, 8G6, and 4C1 as potent antibody candidates for further investigations and pave the way for further developments of therapies and vaccines.
Keywords: Ebola virus; Ebola virus disease; fully human antibody; neutralizing antibodies; surrogate model; transgenic mice.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
