Prognostic value of type of prior TKI in pretreated metastatic renal cell carcinoma patients receiving nivolumab
- PMID: 39155821
- PMCID: PMC11633405
- DOI: 10.1080/1750743X.2024.2385881
Prognostic value of type of prior TKI in pretreated metastatic renal cell carcinoma patients receiving nivolumab
Abstract
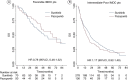
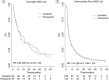
Aim: To define the prognostic significance of first-line TKI in mRCC patients receiving nivolumab.Materials and methods: A total of 571 mRCC patients who received ≥second line nivolumab were included in this subanalysis. The correlation between prior TKI (sunitinib vs. pazopanib) and overall response rate (ORR), disease control rate, progression-free survival and overall survival were investigated. Additionally, the impact of TKI choice according to the International Metastatic RCC Database Consortium prognostic score was examined.Results: There was no significant difference between sunitinib and pazopanib groups in terms of mPFS, mOS, overall response rate and disease control rate. Moreover, no difference between sunitinib and pazopanib was found according to the International Metastatic RCC Database Consortium prognostic score.Conclusion: There is no conclusive evidence favoring pazopanib or sunitinib treatment before initiating nivolumab therapy in metastatic renal cell carcinoma patients.
Keywords: IMDC score; meet-URO score; metastatic renal cell carcinoma; nivolumab; treatment sequence; tyrosine kinase inhibitor.
Plain language summary
[Box: see text].
Conflict of interest statement
S Buti received honoraria as a speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, Roche, Eli Lilly, AstraZeneca, Pierre-Fabre, Novartis, Merck, Gentili, Astellas. G Fornarini services advisory boards for Astellas, Janssen, Pfizer, Bayer, MSD, Merck and received travel accommodation from Astellas, Janssen, Bayer. SE Rebuzzi received honoraria as a speaker at scientific events and travel accommodation from BMS, Amgen, GSK, Ipsen, Astellas, Janssen, MSD. P Rescigno services advisory boards for MSD, AstraZeneca and Janssen. GL Banna reports personal fees from AstraZeneca and Astellas for the speaker bureau. F Morelli received grants from MSD and Pfizer. ***. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/jco.2009.27.15_suppl.5041 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
