Antisense Oligonucleotide Therapy for Calmodulinopathy
- PMID: 39155863
- PMCID: PMC11747850
- DOI: 10.1161/CIRCULATIONAHA.123.068111
Antisense Oligonucleotide Therapy for Calmodulinopathy
Abstract
Background: Calmodulinopathies are rare inherited arrhythmia syndromes caused by dominant heterozygous variants in CALM1, CALM2, or CALM3, which each encode the identical CaM (calmodulin) protein. We hypothesized that antisense oligonucleotide (ASO)-mediated depletion of an affected calmodulin gene would ameliorate disease manifestations, whereas the other 2 calmodulin genes would preserve CaM level and function.
Methods: We tested this hypothesis using human induced pluripotent stem cell-derived cardiomyocyte and mouse models of CALM1 pathogenic variants.
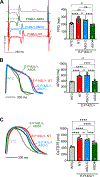
Results: Human CALM1F142L/+ induced pluripotent stem cell-derived cardiomyocytes exhibited prolonged action potentials, modeling congenital long QT syndrome. CALM1 knockout or CALM1-depleting ASOs did not alter CaM protein level and normalized repolarization duration of CALM1F142L/+ induced pluripotent stem cell-derived cardiomyocytes. Similarly, an ASO targeting murine Calm1 depleted Calm1 transcript without affecting CaM protein level. This ASO alleviated drug-induced bidirectional ventricular tachycardia in Calm1N98S/+ mice without a deleterious effect on cardiac electrical or contractile function.
Conclusions: These results provide proof of concept that ASOs targeting individual calmodulin genes are potentially effective and safe therapies for calmodulinopathies.
Keywords: antisense oligonucleotide; calcium; long QT syndrome; precision medicine; tachycardia, ventricular.
Conflict of interest statement
A.E.M. and D.K. are employees of Ionis Pharmaceuticals, which provided the ASOs used in this study. The other authors report no conflicts.
Figures



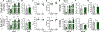
References
-
- Crooke ST, Baker BF, Crooke RM, Liang X-H. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20:427–453. - PubMed
-
- Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, Kuniholm A, Soucy A, Vaze J, Belur NR, Fredriksen K, Stojkovska I, Tsytsykova A, Armant M, DiDonato RL, Choi J, Cornelissen L, Pereira LM, Augustine EF, Genetti CA, Dies K, Barton B, Williams L, Goodlett BD, Riley BL, Pasternak A, Berry ER, Pflock KA, Chu S, Reed C, Tyndall K, Agrawal PB, Beggs AH, Grant PE, Urion DK, Snyder RO, Waisbren SE, Poduri A, Park PJ, Patterson A, Biffi A, Mazzulli JR, Bodamer O, Berde CB, Yu TW. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N Engl J Med. 2019;381:1644–1652. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

