Allogeneic CD4 T Cells Sustain Effective BK Polyomavirus-Specific CD8 T Cell Response in Kidney Transplant Recipients
- PMID: 39156165
- PMCID: PMC11328547
- DOI: 10.1016/j.ekir.2024.04.070
Allogeneic CD4 T Cells Sustain Effective BK Polyomavirus-Specific CD8 T Cell Response in Kidney Transplant Recipients
Abstract
Introduction: BK polyomavirus-associated nephropathy (BKPyVAN) is a significant complication in kidney transplant recipients (KTRs), associated with a higher level of plasmatic BK polyomavirus (BKPyV) replication and leading to poor graft survival.
Methods: We prospectively followed-up with 100 KTRs with various degrees of BKPyV reactivation (no BKPyV reactivation, BKPyV-DNAuria, BKPyV-DNAemia, and biopsy-proven BKPyVAN [bp-BKPyVAN], 25 patients per group) and evaluated BKPyV-specific T cell functionality and phenotype.
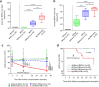
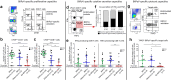
Results: We demonstrate that bp-BKPyVAN is associated with a loss of BKPyV-specific T cell proliferation, cytokine secretion, and cytotoxic capacities. This severe functional impairment is associated with an overexpression of lymphocyte inhibitory receptors (programmed cell death 1 [PD1], cytotoxic T lymphocyte-associated protein 4, T cell immunoreceptor with Ig and ITIM domains, and T cell immunoglobulin and mucin domain-containing-3), highlighting an exhausted-like phenotype of BKPyV-specific CD4 and CD8 T cells in bp-BKPyVAN. This T cell dysfunction is associated with low class II donor-recipient human leukocyte antigen (HLA) divergence. In contrast, in the context of higher class II donor-recipient HLA (D/R-HLA) divergence, allogeneic CD4 T cells can provide help that sustains BKPyV-specific CD8 T cell responses. In vitro, allogeneic HLA-mismatched CD4 T cells rescue BKPyV-specific CD8 T cell responses.
Conclusion: Our findings suggest that in KTRs, allogeneic CD4 T cells can help to maintain an effective BKPyV-specific CD8 T cell response that better controls BKPyV replication in the kidney allograft and may protect against BKPyVAN.
Keywords: BK polyomavirus reactivation; BK polyomavirus-associated nephropathy; BK polyomavirus-specific T cell; donor-recipient HLA divergence; kidney transplantation.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures




References
-
- Ramos E., Drachenberg C.B., Portocarrero M., et al. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin Transpl. 2002:143–153. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

