Outcomes of Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty and Pulmonary Endarterectomy
- PMID: 39156509
- PMCID: PMC11328766
- DOI: 10.1016/j.jacasi.2024.05.007
Outcomes of Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty and Pulmonary Endarterectomy
Abstract
Background: The contemporary outcome of balloon pulmonary angioplasty (BPA) and pulmonary endarterectomy (PEA) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) are unclear.
Objectives: This study aimed to clarify the characteristics and outcomes of CTEPH patients treated with BPA and PEA in Japan.
Methods: Among 1,270 participants enrolled between 2018 and 2023 in the CTEPH AC (Chronic Thromboembolic Pulmonary Hypertension Anticoagulant) registry, a Japanese nationwide CTEPH registry, 369 treatment-naive patients (BPA strategy: n = 313; PEA strategy: n = 56) and 690 on-treatment patients (BPA strategy: n = 561; PEA strategy: n = 129) were classified according to the presence of prior reperfusion therapy. Morbidity and mortality events (all-cause death, rescue mechanical reperfusion therapy, and/or initiation of parenteral pulmonary vasodilators), pulmonary hemodynamics, exercise tolerance, and relevant laboratory test results were evaluated.
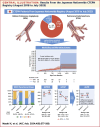
Results: The BPA strategy was chosen in older patients than the PEA strategy (mean age, BPA vs PEA: 66.5 ± 12.6 years vs 62.5 ± 11.8 years; P = 0.028). Median follow-up period was 615 (Q1-Q3: 311-997) days in treatment-naive patients and 1,136 (Q1-Q3: 684-1,300) days in on-treatment patients. BPA strategy had as acceptable morbidity and mortality as PEA strategy (5-year morbidity and mortality event rate, BPA vs PEA: 10.2% [95% CI: 5.2%-19.5%] vs 16.1% [95% CI: 4.3%-50.6%] in treatment-naive patients; 9.7% [95% CI: 6.7%-13.8%] vs 6.9% [95% CI: 2.7%-17.3%] in on-treatment patients), with greater improvement of renal function; glomerular filtration rate in propensity score-matched population (difference between change: 4.9 [95% CI: 0.5-9.3] mL/min/1.73 m2; P = 0.030).
Conclusions: BPA strategy was more frequently chosen in older patients compared with PEA strategy and showed acceptable outcomes for efficacy with greater advantage for improvement in renal function. (Multicenter registry of chronic thromboembolic pulmonary hypertension in Japan; UMIN000033784).
Keywords: pulmonary embolism; pulmonary hypertension; registry; thromboembolism.
© 2024 The Authors.
Conflict of interest statement
This study is supported by the Japan Agency for Medical Research and Development (grant numbers JP20ek0109371, JP19lk0201102, JP22lk0201125, and JP19lk1601003), JSPS KAKENHI (grant number JP20286266), and grant from Konica Minolta based on a contract. The funding body had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Dr Hosokawa has received grants from Konica Minolta; and personal fees from Bayer Yakuhin, Nippon Shinyaku, Janssen Pharmaceutical, Pfizer, and Konica Minolta, outside the submitted work. Dr Taniguchi has received grants from Nippon Shinyaku and Janssen Pharmaceutical; and personal fees from Nippon Shinyaku, Janssen Pharmaceutical, and Bayer Yakuhin, outside the submitted work. Dr Inami has received personal fees from Janssen Pharmaceutical and Bayer Yakuhin, outside the submitted work. Dr Yamashita has received a grant from Abbott Vascular Japan; and personal fees from Kaneka Medix, Boston Scientific Japan, Nihon Kohden, Philips Japan, Janssen Pharmaceutical, and Bayer Yakuhin, outside the submitted work. Dr Ogino has received consulting fees from Terumo, Japan Lifeline, and Century Medical; and personal fees from Bayer Yakuhin, Daiichi-Sankyo, Pfizer, and Nippon Shinyaku, outside the submitted work. Dr Tsujino has received personal fees from Nippon Shinyaku and Janssen Pharmaceutical; and affiliation with the division supported by endowments from Nippon Shinyaku, Nippon Boehringer Ingelheim, Mochida Pharmaceutical, Kaneka Medix, Takeyama, and Medical System Network, outside the submitted work. Dr Hatano has received personal fees from Bayer Yakuhin and Janssen Pharmaceutical, outside the submitted work. Dr Yaoita has received personal fees from Bayer Yakuhin and Konica Minolta, outside the submitted work. Dr Ikeda has received personal fees from Janssen Pharmaceutical, Bayer Yakuhin, Nippon Shinyaku, Daiichi -Sankyo, and Bristol Myers Squibb, outside the submitted work. Dr Shimokawahara has received a grant from Bayer Yakuhin; and personal fees from Bayer Yakuhin and Nippon Shinyaku, outside the submitted work. Dr Tanabe has received personal fees from Janssen Pharmaceutical, Bayer Yakuhin, and Nippon Shinyaku, outside the submitted work. Dr Kubota has received personal fees from Janssen Pharmaceutical and Nippon Shinyaku, outside the submitted work. Dr Ogihara has received grants from Bayer Yakuhin; and personal fees from Janssen Pharmaceutical, Bayer Yakuhin, Nippon Shinyaku, Daiichi-Sankyo, and Bristol Myers Squibb, outside the submitted work. Dr Kawakami has received personal fees from Kaneka Medix and Abbott Medical Japan, and consulting fees from ACIST Japan, outside the submitted work. Dr Tamura has received grants from Bayer Yakuhin, Nippon Shinyaku, and Mochida Pharmaceutical; and personal fees from Bayer Yakuhin, Nippon Shinyaku, Daiichi-Sankyo, and Janssen Pharmaceutical, outside the submitted work. Dr Abe has received a grant from Konica Minolta and Daiichi-Sankyo, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Hoeper M.M., Mayer E., Simonneau G., Rubin L.J. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–2020. - PubMed
-
- Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. - PubMed
-
- Taniguchi Y., Miyagawa K., Nakayama K., et al. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10:518–525. - PubMed
-
- Shimura N., Kataoka M., Inami T., et al. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–142. - PubMed
LinkOut - more resources
Full Text Sources

