Dynamic A-to-I RNA editing during acute neuroinflammation in sepsis-associated encephalopathy
- PMID: 39156629
- PMCID: PMC11328407
- DOI: 10.3389/fnins.2024.1435185
Dynamic A-to-I RNA editing during acute neuroinflammation in sepsis-associated encephalopathy
Abstract
Introduction: The activation of cerebral endothelial cells (CECs) has recently been reported to be the earliest acute neuroinflammation event in the CNS during sepsis-associated encephalopathy (SAE). Importantly, adenosine-to-inosine (A-to-I) RNA editing mediated by ADARs has been associated with SAE, yet its role in acute neuroinflammation in SAE remains unclear.
Methods: Our current study systematically analyzed A-to-I RNA editing in cerebral vessels, cerebral endothelial cells (CECs), and microglia sampled during acute neuroinflammation after treatment in a lipopolysaccharide (LPS)-induced SAE mouse model.
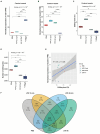
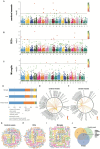
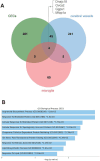
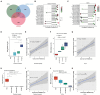
Results: Our results showed dynamic A-to-I RNA editing activity changes in cerebral vessels during acute neuroinflammation. Differential A-to-I RNA editing (DRE) associated with acute neuroinflammation were identified in these tissue or cells, especially missense editing events such as S367G in antizyme inhibitor 1 (Azin1) and editing events in lincRNAs such as maternally expressed gene 3 (Meg3), AW112010, and macrophage M2 polarization regulator (Mm2pr). Importantly, geranylgeranyl diphosphate synthase 1 (Ggps1) and another three genes were differentially edited across cerebral vessels, CECs, and microglia. Notably, Spearman correlation analysis also revealed dramatic time-dependent DRE during acute neuroinflammation, especially in GTP cyclohydrolase1 (Gch1) and non-coding RNA activated by DNA damage (Norad), both with the editing level positively correlated with both post-LPS treatment time and edited gene expression in cerebral vessels and CECs.
Discussion: The findings in our current study demonstrate substantial A-to-I RNA editing changes during acute neuroinflammation in SAE, underlining its potential role in the disease.
Keywords: RNA editing; acute neuroinflammation; cerebral endothelial cells; cerebral vessels; microglia; sepsis-associated encephalopathy.
Copyright © 2024 Li, Liang, Zhang, Li, Wei, Rao, Chen and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer QL declared a past co-authorship with the author J-HC to the handling editor. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Temporal unsnarling of brain's acute neuroinflammatory transcriptional profiles reveals panendothelitis as the earliest event preceding microgliosis.Mol Psychiatry. 2021 Aug;26(8):3905-3919. doi: 10.1038/s41380-020-00955-5. Epub 2020 Dec 8. Mol Psychiatry. 2021. PMID: 33293688 Free PMC article.
-
Bulk and single-cell RNA-seq analyses reveal canonical RNA editing associated with microglia homeostasis and its role in sepsis-associated encephalopathy.Neuroscience. 2024 Nov 12;560:167-180. doi: 10.1016/j.neuroscience.2024.09.027. Epub 2024 Sep 16. Neuroscience. 2024. PMID: 39293730
-
Hippocampal adenosine-to-inosine RNA editing in sepsis: dynamic changes and influencing factors.Brain Commun. 2024 Aug 8;6(4):fcae260. doi: 10.1093/braincomms/fcae260. eCollection 2024. Brain Commun. 2024. PMID: 39135964 Free PMC article.
-
Central role of microglia in sepsis-associated encephalopathy: From mechanism to therapy.Front Immunol. 2022 Jul 26;13:929316. doi: 10.3389/fimmu.2022.929316. eCollection 2022. Front Immunol. 2022. PMID: 35958583 Free PMC article. Review.
-
Sepsis-associated encephalopathy: Autophagy and miRNAs regulate microglial activation.Physiol Rep. 2024 Mar;12(5):e15964. doi: 10.14814/phy2.15964. Physiol Rep. 2024. PMID: 38439741 Free PMC article. Review.
Cited by
-
GAS6/AXL signaling promotes M2 microglia efferocytosis to alleviate neuroinflammation in sepsis-associated encephalopathy.Cell Death Discov. 2025 Jun 6;11(1):268. doi: 10.1038/s41420-025-02507-8. Cell Death Discov. 2025. PMID: 40480981 Free PMC article.
References
-
- Antoniades C., Cunnington C., Antonopoulos A., Neville M., Margaritis M., Demosthenous M., et al. . (2011). Induction of vascular GTP-Cyclohydrolase I and endogenous tetrahydrobiopterin synthesis protect against inflammation-induced endothelial dysfunction in human atherosclerosis. Circulation 124, 1860–1870. doi: 10.1161/CIRCULATIONAHA.111.029272, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

