Fission yeast Bgs1 glucan synthase participates in the control of growth polarity and membrane traffic
- PMID: 39156640
- PMCID: PMC11326927
- DOI: 10.1016/j.isci.2024.110477
Fission yeast Bgs1 glucan synthase participates in the control of growth polarity and membrane traffic
Abstract
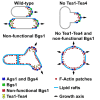
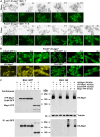
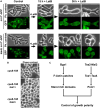
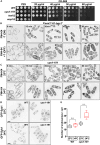
Rod-shaped fission yeast grows through cell wall expansion at poles and septum, synthesized by essential glucan synthases. Bgs1 synthesizes the linear β(1,3)glucan of primary septum at cytokinesis. Linear β(1,3)glucan is also present in the wall poles, suggesting additional Bgs1 roles in growth polarity. Our study reveals an essential collaboration between Bgs1 and Tea1-Tea4, but not other polarity factors, in controlling growth polarity. Simultaneous absence of Bgs1 function and Tea1-Tea4 causes complete loss of growth polarity, spread of other glucan synthases, and spherical cell formation, indicating this defect is specifically due to linear β(1,3)glucan absence. Furthermore, linear β(1,3)glucan absence induces actin patches delocalization and sterols spread, which are ultimately responsible for the growth polarity loss without Tea1-Tea4. This suggests strong similarities in Bgs1 functions controlling actin structures during cytokinesis and polarized growth. Collectively, our findings unveil that cell wall β(1,3)glucan regulates polarized growth, like the equivalent extracellular matrix in neuronal cells.
Keywords: Functional aspects of cell biology; Mycology; Organizational aspects of cell biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination.J Cell Sci. 2002 Nov 1;115(Pt 21):4081-96. doi: 10.1242/jcs.00085. J Cell Sci. 2002. PMID: 12356913
-
Sbg1 Is a Novel Regulator for the Localization of the β-Glucan Synthase Bgs1 in Fission Yeast.PLoS One. 2016 Nov 29;11(11):e0167043. doi: 10.1371/journal.pone.0167043. eCollection 2016. PLoS One. 2016. PMID: 27898700 Free PMC article.
-
Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast.PLoS Genet. 2015 Jul 1;11(7):e1005358. doi: 10.1371/journal.pgen.1005358. eCollection 2015 Jul. PLoS Genet. 2015. PMID: 26132084 Free PMC article.
-
Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe.Fungal Genet Biol. 1998 Jun-Jul;24(1-2):178-206. doi: 10.1006/fgbi.1998.1067. Fungal Genet Biol. 1998. PMID: 9742201 Review.
-
Microtubule-dependent cell morphogenesis in the fission yeast.Trends Cell Biol. 2009 Sep;19(9):447-54. doi: 10.1016/j.tcb.2009.06.003. Epub 2009 Aug 25. Trends Cell Biol. 2009. PMID: 19713114 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

