Monitoring GPCR conformation with GFP-inspired dyes
- PMID: 39156645
- PMCID: PMC11326922
- DOI: 10.1016/j.isci.2024.110466
Monitoring GPCR conformation with GFP-inspired dyes
Abstract
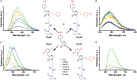
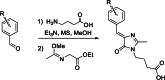
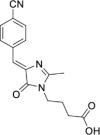
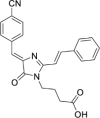
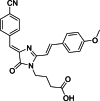
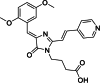
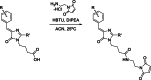
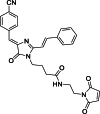
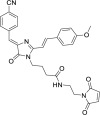
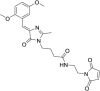
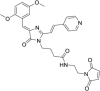
Solvatochromic compounds have emerged as valuable environment-sensitive probes for biological research. Here we used thiol-reactive solvatochromic analogs of the green fluorescent protein (GFP) chromophore to track conformational changes in two proteins, recoverin and the A2A adenosine receptor (A2AAR). Two dyes showed Ca2+-induced fluorescence changes when attached to recoverin. Our best-performing dye, DyeC, exhibited agonist-induced changes in both intensity and shape of its fluorescence spectrum when attached to A2AAR; none of these effects were observed with other common environment-sensitive dyes. Molecular dynamics simulations showed that activation of the A2AAR led to a more confined and hydrophilic environment for DyeC. Additionally, an allosteric modulator of A2AAR induced distinct fluorescence changes in the DyeC spectrum, indicating a unique receptor conformation. Our study demonstrated that GFP-inspired dyes are effective for detecting structural changes in G protein-coupled receptors (GPCRs), offering advantages such as intensity-based and ratiometric tracking, redshifted fluorescence spectra, and sensitivity to allosteric modulation.
Keywords: Biochemistry; Biophysics; Structural biology.
© 2024 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Zimmer M. Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem. Rev. 2002;102:759–781. - PubMed
-
- Walker C.L., Lukyanov K.A., Yampolsky I.V., Mishin A.S., Bommarius A.S., Duraj-Thatte A.M., Azizi B., Tolbert L.M., Solntsev K.M. Fluorescence imaging using synthetic GFP chromophores. Curr. Opin. Chem. Biol. 2015;27:64–74. - PubMed
-
- Smirnov A.Y., Perfilov M.M., Zaitseva E.R., Zagudaylova M.B., Zaitseva S.O., Mishin A.S., Baranov M.S. Design of red-shifted and environment-sensitive fluorogens based on GFP chromophore core. Dyes Pigm. 2020;177
-
- Ermakova Y.G., Sen T., Bogdanova Y.A., Smirnov A.Y., Baleeva N.S., Krylov A.I., Baranov M.S. Pyridinium Analogues of Green Fluorescent Protein Chromophore: Fluorogenic Dyes with Large Solvent-Dependent Stokes Shift. J. Phys. Chem. Lett. 2018;9:1958–1963. - PubMed
-
- Perfilov M.M., Zaitseva E.R., Smirnov A.Y., Mikhaylov A.A., Baleeva N.S., Myasnyanko I.N., Mishin A.S., Baranov M.S. Environment-sensitive fluorogens based on a GFP chromophore structural motif. Dyes Pigm. 2022;198
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

