One-Step Physical and Chemical Dual-Reinforcement with Hydrophobic Drug Delivery in Gelatin Hydrogels for Antibacterial Wound Healing
- PMID: 39157075
- PMCID: PMC11325409
- DOI: 10.1021/acsomega.4c01963
One-Step Physical and Chemical Dual-Reinforcement with Hydrophobic Drug Delivery in Gelatin Hydrogels for Antibacterial Wound Healing
Abstract
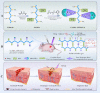
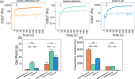
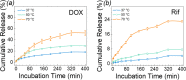
Gelatin-based bioadhesives, especially methacrylated gelatin (GelMA), have emerged as superior alternatives to sutureless wound closure. Nowadays, their mechanical improvement and therapeutic delivery, particularly for hydrophobic antibiotics, have received ever-increasing interest. Herein, a reinforced gelatin-based hydrogel with a hydrophobic drug delivery property for skin wound treatment was reported. First, photosensitive monomers of N'-(2-nitrobenzyl)-N-acryloyl glycinamide (NBNAGA) were grafted onto GelMA via Michael addition, namely, GelMA-NBNAGA. Second, gelation of the GelMA-NBNAGA solution was accomplished in a few seconds under one step of ultraviolet (UV) light irradiation. Multiple effects were realized simultaneously, including chemical cross-linking initiated by lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), physical cross-linking of uncaged dual hydrogen bonding, and hydrophobic drug release along with o-NB group disintegration. The mechanical properties of the dual-reinforcement hydrogels were verified to be superior to those only with a chemical or physical single-cross-linked network. The hydrophobic anticancer doxorubicin (DOX) and antibiotic rifampicin (Rif) were successfully charged into the hydrogels, separately. The in vitro antimicrobial tests confirmed the antibacterial activity of the hydrogels against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria. The in vivo wound-healing assessment in mice further assured their drug release and efficacy. Therefore, this NBNAGA-modified GelMA hydrogel has potential as a material in skin wound dressing with a hydrophobic antibiotic on-demand delivery.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Elias P. M.; Ferngold K. R.. Skin Barrier; CRC Press, 2006.
-
- Montagna W.Structure and Function of Skin; Elsevier, 2012.
-
- Saghazadeh S.; Rinoldi C.; Schot M.; Kashaf S. S.; Sharifi F.; Jalilian E.; Nuutila K.; Giatsidis G.; Mostafalu P.; Derakhshandeh H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Delivery Rev. 2018, 127, 138–166. 10.1016/j.addr.2018.04.008. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
