Preparation, acid modification and catalytic activity of kaolinite nanotubes in α-pinene oxide isomerization
- PMID: 39157207
- PMCID: PMC11328512
- DOI: 10.1039/d4ra03777d
Preparation, acid modification and catalytic activity of kaolinite nanotubes in α-pinene oxide isomerization
Abstract
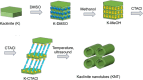
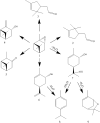
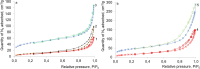
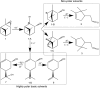
In this work kaolinite nanotubes (KNT) were obtained from commercial kaolin AKF-78 (Uzbekistan) by starting material sequential intercalation by DMSO and methanol, followed by treatment with a cetyltrimethylammonium chloride solution. Acid functionalization of KNT for catalytic applications was successfully performed for the first time using a two-step treatment with piranha solution (H2SO4-H2O2), which resulted in the removal of organic impurities as synthetic artifacts and an increase in specific surface area by 3.9 times (up to 159 m2 g-1), pore volume by 1.5 times (0.23 cm3 g-1) and acidity by 4.1 times (49 μmol g-1). The values of the porous structure parameters and concentration of acid sites in processed kaolinite nanotubes practically corresponded to those for natural halloysite nanotubes (HNT) modified in the same way. Both types of materials demonstrated catalytic activity in the model reaction of α-pinene oxide isomerization in various solvents, including green ones, with selectivity to trans-carveol up to 55-57% and campholenic aldehyde of 50-51%, depending on the medium used. A satisfactory correlation between solvent polarity and selectivity was also observed. To the best of our knowledge, this is the first example of using modified kaolinite nanotubes per se as a catalyst. Overall, treatment of KNT with piranha solution provides not only catalytic activity but also the opportunity for further functionalization and application of these nanomaterials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Fahimizadeh M. Wong L. W. Baifa Z. Sadjadi S. Auckloo S. A. B. Palaniandy K. Pasbakhsh P. Tan J. B. L. Singh R. K. R. Yuan P. Halloysite clay nanotubes: Innovative applications by smart systems. Appl. Clay Sci. 2024;251:107319. doi: 10.1016/j.clay.2024.107319. doi: 10.1016/j.clay.2024.107319. - DOI - DOI
-
- Sidorenko A. Y. Kravtsova A. V. Aho A. Heinmaa I. Warna J. Pazniak H. Volcho K. P. Salakhutdinov N. F. Murzin D. Y. Agabekov V. E. Highly selective Prins reaction over acid-modified halloysite nanotubes for synthesis of isopulegol-derived 2H-chromene compounds. J. Catal. 2019;374:360–377. doi: 10.1016/j.jcat.2019.05.009. doi: 10.1016/j.jcat.2019.05.009. - DOI - DOI
LinkOut - more resources
Full Text Sources

