Novel insights into post-marketing AEs associated with leuprorelin: A comprehensive analysis utilizing the FAERS database
- PMID: 39157412
- PMCID: PMC11327563
- DOI: 10.1016/j.heliyon.2024.e34969
Novel insights into post-marketing AEs associated with leuprorelin: A comprehensive analysis utilizing the FAERS database
Abstract
Purpose: This research focused on meticulously tracking and identifying adverse reactions associated with leuprorelin, a drug prescribed for conditions such as prostate cancer, endometriosis, uterine fibroids, and early-onset puberty. The main objective was to enhance patient safety and offer informed guidance on the appropriate use of this treatment.
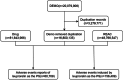
Methods: From the first quarter of 2004 to the fourth quarter of 2023, a comprehensive analysis was conducted on a significant number of adverse event reports (AERs) from the FDA Adverse Event Reporting System (FAERS) database. Data mining with dismutation analysis was conducted to quantify signals associated with adverse events (AEs) related to leuprorelin, utilizing powerful algorithms such as ROR, PRR, BCPNN, and EBGM.
Results: A total of 102 positive reaction terms (PT) spanning 24 System Organ Classes (SOCs) were identified from an analysis of 60,709 reports associated with leuprorelin use. Notably, several previously unrecognized adverse reactions were uncovered, including Artificial Menopause, Ovarian Adhesion, Follicular Cystitis, Intercepted product preparation error, among others. These findings underscore the importance of exercising additional vigilance regarding the potential adverse effects of leuprorelin, such as Abscess Sterile, Injection site granuloma, Intercepted medication error, and Bulbospinal muscular atrophy congenital.
Conclusions: This research has successfully uncovered new and unforeseen signals associated with adverse drug reactions (ADRs) following leuprorelin administration. The study provides valuable insights into the intricate connection between ADRs and leuprorelin usage. The results underscore the crucial significance of continuous surveillance and meticulous monitoring to promptly identify and manage AEs, ultimately enhancing patient safety and well-being while undergoing leuprorelin therapy.
Keywords: BCPNN; EBGM; FAERS; Leuprorelin; PRR; ROR.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Novel insights into post-marketing adverse events associated with lenvatinib: A comprehensive analysis utilizing the FAERS database.Heliyon. 2024 Mar 13;10(6):e28132. doi: 10.1016/j.heliyon.2024.e28132. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524578 Free PMC article.
-
Comprehensive evaluation of leuprorelin-associated adverse events: insights from FDA adverse event reporting system.Expert Opin Drug Saf. 2025 Mar;24(3):355-364. doi: 10.1080/14740338.2024.2423680. Epub 2024 Oct 31. Expert Opin Drug Saf. 2025. PMID: 39469972
-
Comparing adverse events of tenecteplase and alteplase: a real-world analysis of the FDA adverse event reporting system (FAERS).Expert Opin Drug Saf. 2024 Feb;23(2):221-229. doi: 10.1080/14740338.2023.2245745. Epub 2023 Aug 18. Expert Opin Drug Saf. 2024. PMID: 37554093
-
A disproportionality analysis of low molecular weight heparin in the overall population and in pregnancy women using the FDA adverse event reporting system (FAERS) database.Front Pharmacol. 2024 Aug 12;15:1442002. doi: 10.3389/fphar.2024.1442002. eCollection 2024. Front Pharmacol. 2024. PMID: 39188956 Free PMC article.
-
Data mining of the public version of the FDA Adverse Event Reporting System.Int J Med Sci. 2013 Apr 25;10(7):796-803. doi: 10.7150/ijms.6048. Print 2013. Int J Med Sci. 2013. PMID: 23794943 Free PMC article. Review.
References
-
- Wilson A.C., Meethal S.V., Bowen R.L., Atwood C.S. Leuprolide acetate: a drug of diverse clinical applications. Expet Opin. Invest. Drugs. 2007;16(11):1851–1863. - PubMed
-
- Uzunova A.D., Ramey E.R., Ramwell P.W. Gonadal hormones and pathogenesis of occlusive arterial thrombosis. Am. J. Physiol. 1978;234(4):H454–H459. - PubMed
-
- Gillatt D. Antiandrogen treatments in locally advanced prostate cancer: are they all the same? J Cancer Res Clin. 2006;132(Suppl 1):S17–S26. - PubMed
-
- Hoffmann G., Spitz J., Behrens R. [GnRH-agonists in the therapy of endometriosis] Ther. Umsch. 1990;47(12):937–944. - PubMed
-
- Ayaz M., Ullah F., Sadiq A., Ullah F., Ovais M., Ahmed J., Devkota H.P. Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019;308:294–303. - PubMed
LinkOut - more resources
Full Text Sources