Establishing the Safety and Efficacy of Bedaquiline-Containing Regimen for the Treatment of Drug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
- PMID: 39157539
- PMCID: PMC11329311
- DOI: 10.1155/2024/5542658
Establishing the Safety and Efficacy of Bedaquiline-Containing Regimen for the Treatment of Drug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
The risks and benefits of bedaquiline (BDQ) for treatment of drug-resistant tuberculosis (DR-TB) have not been firmly established. We aimed to assess the safety and efficacy of BDQ-containing regimens for the treatment of DR-TB as evidenced in available randomized controlled trials (RCTs). In this systematic review and meta-analysis, five databases (i.e., ClinicalTrials.gov, Cochrane CENTRAL, PubMed, ScienceDirect, and SinoMed) were searched. RCTs among DR-TB patients that had a control arm were eligible. The safety endpoints were all-cause mortality and serious adverse effects (SAEs). Efficacy outcomes were sputum culture conversion rate at 8-12 weeks and 24-26 weeks, treatment success, and time to culture conversion. A total of 476 records were screened; 18 met the eligibility criteria. The pooled analysis included 2520 participants (55.8% received BDQ-containing regimens, n = 1408). Pooled safety outcomes showed no significant reduction in all-cause mortality (relative risk [RR] [95%confidence interval (CI)] = 0.94 [0.41-2.20]) or SAEs (RR [95%CI] = 0.91 [0.67-1.23]) in the BDQ-regimen group. Pooled efficacy outcomes showed significantly superior culture conversion rates at 8-12 weeks (RR [95%CI] = 1.35 [1.10-1.65]) and 24-26 weeks (RR [95%CI] = 1.25 [1.15-1.36]), more treatment success (RR [95%CI] = 1.30 [1.17-1.44]), and a 17-day reduction in the time to culture conversion (standardized mean difference [SMD] [95%CI] = -17.46 [-34.82 to -0.11]) in the BDQ-regimen group (reference: non-BDQ regimen). Overall, BDQ regimens showed significant treatment effect against DR-TB but did not reduce mortality or SAEs.
Keywords: bedaquiline; diarylquinolines; drug-resistant tuberculosis; meta-analysis; systematic review; tuberculosis.
Copyright © 2024 Muhammad Candragupta Jihwaprani et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
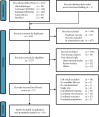
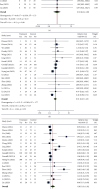
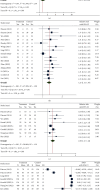
References
-
- World Health Organization. Global tuberculosis report 2023 . Geneva: World Health Organization; 2023. https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf... .
-
- Zürcher K., Reichmuth M. L., Ballif M., et al. Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: a multicentre cohort study. The Lancet Microbe . 2021;2(7):e320–e330. doi: 10.1016/S2666-5247(21)00044-6. - DOI - PMC - PubMed
-
- World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: Interim policy guidance . Geneva: World Health Organization; 2013. https://www.who.int/publications/i/item/9789241505482 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

