Variation in immunoglobulin use and impact on survival in myeloma
- PMID: 39157592
- PMCID: PMC11327709
- DOI: 10.1002/jha2.938
Variation in immunoglobulin use and impact on survival in myeloma
Abstract
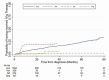
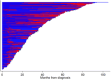
Serious infection is common in patients with multiple myeloma due to immune deficiency from the underlying disease and/or its treatment. Immunoglobulin replacement is one approach to reduce infection risk in these patients. However, few real-world data exist on its use in patients with myeloma. We investigated immunoglobulin use in Australia, New Zealand and Asia-Pacific using registry data and explored its association with survival outcomes. A total of 2374 patients with a median follow-up time of 29.5 months (interquartile range 13.3-54.3 months) were included in the analysis - 1673 from Australia, 313 Korea, 281 New Zealand and 107 Singapore. Overall, 7.1% of participants received immunoglobulin replacement within 24 months of diagnosis. Patients who received immunoglobulin replacement were likely to be younger, had lower baseline IgG levels (excluding paraprotein), were more likely to have baseline hypogammaglobulinaemia, baseline severe hypogammaglobulinaemia and abnormal baseline fluorescent in-situ hybridisation status, receive first-line myeloma treatment with immunomodulatory drugs or anti-CD38 therapy and undergo upfront autologous stem cell transplant. In our patient cohort, the use of immunoglobulin was not associated with overall survival benefit at the time of last follow-up (adjusted hazard ratio 0.72, 95% CI 0.46-1.14, p = 0.16). Understanding treatment approaches in clinical practice can help support future planning and provision of immunoglobulin resources.
Keywords: immunoglobulin; infection; multiple myeloma.
© 2024 The Author(s). eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
This research project did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. However, the ANZ MRDR has received funding from Abbvie, Amgen, Antengene, Bristol‐Myers Squibb, Celgene, Gilead, GSK, Janssen, Novartis, Sanofi and Takeda. The APAC MRDR has received funding from Janssen Asia‐Pacific. Monash University has received funding from CSL Behring for other projects.
Figures
References
-
- Wyndham A, Vogan A, Newton S, Schubert C. Immunoglobulin for acquired hypogammaglobulinaemia secondary to haematological malignancies, or post‐haemopoietic stem cell transplantation. MSAC Assessment Report 2019. Commonwealth of Australia, Canberra: ACT; 2019.
-
- Raanani P, Gafter‐Gvili A, Paul M, Ben‐Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta‐analysis. Leuk Lymphoma. 2009;50(5):764–772. - PubMed
-
- Blombery P, Prince HM, Worth LJ, Main J, Yang M, Wood EM, et al. Prophylactic intravenous immunoglobulin during autologous haemopoietic stem cell transplantation for multiple myeloma is not associated with reduced infectious complications. Ann Hematol. 2011;90(10):1167–1172. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
