iRGD-Guided Silica/Gold Nanoparticles for Efficient Tumor-Targeting and Enhancing Antitumor Efficacy Against Breast Cancer
- PMID: 39157735
- PMCID: PMC11329605
- DOI: 10.2147/IJN.S474135
iRGD-Guided Silica/Gold Nanoparticles for Efficient Tumor-Targeting and Enhancing Antitumor Efficacy Against Breast Cancer
Abstract
Background: Breast cancer presents significant challenges due to the limited effectiveness of available treatments and the high likelihood of recurrence. iRGD possesses both RGD sequence and C-terminal sequence and has dual functions of targeting and membrane penetration. iRGD-modified nanocarriers can enhance drug targeting of tumor vascular endothelial cells and penetration of new microvessels, increasing drug concentration in tumor tissues.
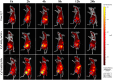
Methods: The amidation reaction was carried out between SiO2/AuNCs and iRGD/PTX, yielding a conjugated drug delivery system (SiO2/AuNCs-iRGD/PTX, SAIP@NPs). The assessment encompassed the characterization of the morphology, particle size distribution, physicochemical properties, in vitro release profile, cytotoxicity, and cellular uptake of SAIP@NPs. The tumor targeting and anti-tumor efficacy of SAIP@NPs were assessed using a small animal in vivo imaging system and a tumor-bearing nude mice model, respectively. The tumor targeting and anti-tumor efficacy of SAIP@NPs were assessed utilizing a small animal in vivo imaging system and an in situ nude mice breast cancer xenograft model, respectively.
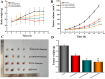
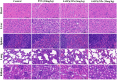
Results: The prepared SAIP@NPs exhibited decent stability and a certain slow-release effect in phosphate buffer (PBS, pH 7.4). In vitro studies had shown that, due to the dual functions of transmembrane and targeting of iRGD peptide, SAIP@NPs exhibited strong binding to integrin αvβ3, which was highly expressed on the membrane of MDA-MB-231 cells, improving the uptake capacity of tumor cells, inhibiting the rapid growth of tumor cells, and promoting tumor cell apoptosis. The results of animal experiments further proved that SAIP@NPs had longer residence time in tumor sites, stronger anti-tumor effect, and no obvious toxicity to major organs of experimental animals.
Conclusion: The engineered SAIP@NPs exhibited superior functionalities including efficient membrane permeability, precise tumor targeting, and imaging, thereby significantly augmenting the therapeutic efficacy against breast cancer with a favorable safety profile.
Keywords: breast cancer; gold nanoclusters; iRGD penetrating peptide; integrin αvβ3; silicon dioxide nanoparticles; tumor targeting.
© 2024 Hou et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

