Coagulation factor XI regulates endothelial cell permeability and barrier function in vitro and in vivo
- PMID: 39158072
- PMCID: PMC11830974
- DOI: 10.1182/blood.2023022257
Coagulation factor XI regulates endothelial cell permeability and barrier function in vitro and in vivo
Abstract
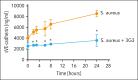
Loss of endothelial barrier function contributes to the pathophysiology of many inflammatory diseases. Coagulation factor XI (FXI) plays a regulatory role in inflammation. Although activation of FXI increases vascular permeability in vivo, the mechanism by which FXI or its activated form FXIa disrupts endothelial barrier function is unknown. We investigated the role of FXIa in human umbilical vein endothelial cell (HUVEC) or human aortic endothelial cell (HAEC) permeability. The expression patterns of vascular endothelial (VE)-cadherin and other proteins of interest were examined by western blot or immunofluorescence. Endothelial cell permeability was analyzed by Transwell assay. We demonstrate that FXIa increases endothelial cell permeability by inducing cleavage of the VE-cadherin extracellular domain, releasing a soluble fragment. The activation of a disintegrin and metalloproteinase 10 (ADAM10) mediates the FXIa-dependent cleavage of VE-cadherin, because adding an ADAM10 inhibitor prevented the cleavage of VE-cadherin induced by FXIa. The binding of FXIa with plasminogen activator inhibitor 1 and very low-density lipoprotein receptor on HUVEC or HAEC surfaces activates vascular endothelial growth receptor factor 2 (VEGFR2). The activation of VEGFR2 triggers the mitogen-activated protein kinase (MAPK) signaling pathway and promotes the expression of active ADAM10 on the cell surface. In a pilot experiment using an established baboon model of sepsis, the inhibition of FXI activation significantly decreased the levels of soluble VE-cadherin to preserve barrier function. This study reveals a novel pathway by which FXIa regulates vascular permeability. The effect of FXIa on barrier function may be another way by which FXIa contributes to the development of inflammatory diseases.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: C.U.L. and E.I.T. are employees of Aronora Inc, a company that may have a commercial interest in the results of this research. J.J.S. serves as a medical consultant for Aronora, Inc; this potential conflict of interest has been reviewed and managed by the Oregon Health and Science University conflict of interest in research committee. The remaining authors declare no competing financial interests.
Figures


References
-
- Wettschureck N, Strilic B, Offermanns S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 2019;99(3):1467–1525. - PubMed
-
- Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

