Peripheral nerve injury results in a biased loss of sensory neuron subpopulations
- PMID: 39158319
- PMCID: PMC11562755
- DOI: 10.1097/j.pain.0000000000003321
Peripheral nerve injury results in a biased loss of sensory neuron subpopulations
Abstract
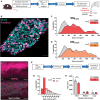
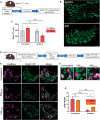
There is a rich literature describing the loss of dorsal root ganglion (DRG) neurons following peripheral axotomy, but the vulnerability of discrete subpopulations has not yet been characterised. Furthermore, the extent or even presence of neuron loss following injury has recently been challenged. In this study, we have used a range of transgenic recombinase driver mouse lines to genetically label molecularly defined subpopulations of DRG neurons and track their survival following traumatic nerve injury. We find that spared nerve injury leads to a marked loss of cells containing DRG volume and a concomitant loss of small-diameter DRG neurons. Neuron loss occurs unequally across subpopulations and is particularly prevalent in nonpeptidergic nociceptors, marked by expression of Mrgprd. We show that this subpopulation is almost entirely lost following spared nerve injury and severely depleted (by roughly 50%) following sciatic nerve crush. Finally, we used an in vitro model of DRG neuron survival to demonstrate that nonpeptidergic nociceptor loss is likely dependent on the absence of neurotrophic support. Together, these results profile the extent to which DRG neuron subpopulations can survive axotomy, with implications for our understanding of nerve injury-induced plasticity and pain.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
D.L.B. has acted as a consultant in the last 2 years for AditumBio, Biogen, Biointervene, Combigene, LatigoBio, GSK, Ionis, Lexicon therapeutics, Neuvati, Olipass, Orion, Replay, SC Health Managers, Theranexus, Third Rock Ventures, and Vida Ventures on behalf of Oxford University Innovation. D.L.B. has received research funding from Lilly and Astra Zeneca, and G.A.W. has received research funding from Ono Pharmaceutical. D.L.B. has received an industrial partnership grant from the BBSRC and AstraZeneca. The remaining authors have no conflicts of interest to declare.
Data are available on request to lead contact G.A.W.—
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O'Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 2017;168:295–310.e19. - PMC - PubMed
-
- Bailey AL, Ribeiro-Da-Silva A. Transient loss of terminals from non-peptidergic nociceptive fibers in the substantia gelatinosa of spinal cord following chronic constriction injury of the sciatic nerve. Neuroscience 2006;138:675–90. - PubMed
-
- Bell AM, Utting C, Dickie AC, Kucharczyk MW, Quillet R, Gutierrez-Mecinas M, Razlan ANB, Cooper AH, Lan Y, Hachisuka J, Weir GA, Bannister K, Watanabe M, Kania A, Hoon MA, Macaulay IC, Denk F, Todd AJ. Deep sequencing of Phox2a nuclei reveals five classes of anterolateral system neurons. bioRxiv 2023.2023.08.20.553715. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

